The analysis of different types of corrosion is essential for predicting damage and applying appropriate prevention methods in different environments and materials, predictive monitoring, and comprehensive industrial asset management. Since corrosion is responsible for significant economic losses and critical equipment failures. Corrosion can be classified in different ways: according to the medium in which it occurs (chemical or electrochemical), according to its appearance (uniform or localized, such as pitting or galvanic corrosion), and also by the influence of external factors, such as mechanical stress or the action of microorganisms.
This article will address the technical classification proposed by Fontana, which distinguishes eight types of corrosion based on their form and appearance. This classification is one of the most widely accepted in the scientific community, although it varies slightly from the definitions of other authors.
How is corrosion classified?
According to the above, corrosion can be classified according to the mechanism of attack, its appearance, and the environment and factors involved in the process. Considering Fontana’s classification according to appearance, the following types can be distinguished: Generalized corrosion: (1) uniform corrosion; and localized corrosion, which includes: (2) galvanic or dissimilar metal corrosion, (3) crevice corrosion, (4) pitting, (5) intergranular corrosion, (6) selective leaching or desaltation, and (7) stress corrosion. This list is arbitrary, but it covers virtually all corrosion failures and problems.
- Generalized corrosion: This occurs uniformly across the metal surface, making it easy to measure and monitor through periodic inspections.
- Localized corrosion: In contrast, it is concentrated in specific areas of the material, such as cracks, edges, or joints between different metals, or areas with limited oxygen or confined spaces. It is often more dangerous, as it is more difficult to detect and poses a greater risk to structural safety due to its hidden and rapid development.
What is generalized corrosion, and how can it be prevented?
1. Uniform corrosion

This type of corrosion is the most common, causing a progressive decrease in thickness. It generally occurs in environments where the material is in contact with aggressive media such as acids, alkalis, or water.
Prevention: It is recommended to use materials with high corrosion resistance, such as stainless steels or aluminum alloys, in addition to performing regular cleaning and maintenance, avoiding prolonged exposure to corrosive agents. This may include the application of protective coatings, appropriate design, or the implementation of cathodic and anodic protection techniques.
What is localized corrosion, types, and how to prevent it?
It is a type of electrochemical degradation of metal that does not uniformly affect the entire exposed surface, but rather concentrates in specific and limited areas, causing severe damage to specific points of the material. It occurs mainly in confined spaces and aggressive environments where heterogeneous conditions exist, such as variations in oxygen concentration, the presence of chlorides, deposits or incrustations, and discontinuities in protective coatings.
The prevention of each type of corrosion is based on a combination of material selection, proper design, environmental control, and protection techniques. Its control depends on the integration of metallurgical, design, environmental, and maintenance practices, ensuring the integrity of critical assets such as pipelines, pressure vessels, marine equipment, and heat exchangers. The types and recommendations for their control are described below.
2. Galvanic corrosion

The severity of the attack depends on factors such as the relative position of the materials in the galvanic series, the resistivity of the electrolyte, and, critically, the area ratio between the anode and cathode. A small anodic surface coupled with a large cathodic surface results in high current density on the anode, causing rapid material loss in that area.
Prevention: Direct contact between different metals should be avoided, or, failing that, coatings should be used or the metals should be insulated to interrupt electrical conductivity. It is advisable to use metals with similar electrochemical potentials, so as to minimize the potential difference and, consequently, the rate of galvanic corrosion.
3. Crevice corrosion
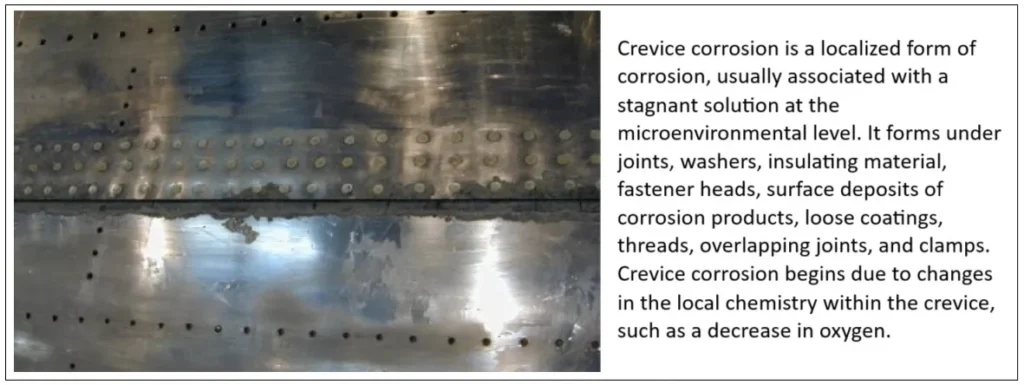
It occurs when oxygen diffusion in the crack decreases, tending to form a differential aeration cell between the crack (microenvironment) and the external surface (global environment).
Prevention: Avoid the formation of holes or discontinuities in the metal surface by designing joints appropriately and carrying out regular inspections and cleaning to prevent moisture or water from accumulating in these susceptible areas.
4. Pitting corrosion

Pitting corrosion generally occurs in aggressive environments such as seawater, chloride solutions, or media with low dissolved oxygen content.
Prevention: Use high-strength alloys, such as high-alloy stainless steels, and supplement with coatings.
5. Intergranular and transgranular corrosion
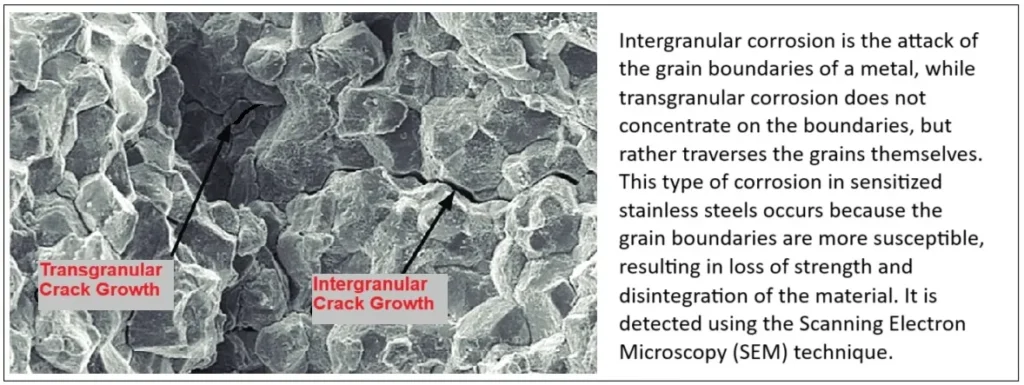
The metallic microstructure is composed of crystalline grains delimited by these boundaries, which can act as preferential corrosion zones due to chemical or structural differences with respect to the interior of the grains.
Prevention: Avoid its main cause: the precipitation of chromium carbides at grain boundaries, which is achieved by using low-carbon steels (type “L” steel), adding stabilizing elements such as titanium or niobium, and appropriate heat treatment that includes heating to temperatures above 1100 °C and rapid cooling.
6. Stress Corrosion Cracking (SCC)

Stress corrosion cracking refers to cracking caused by the simultaneous presence of tensile stress and a specific corrosive medium. Many researchers have classified all cracking failures that occur in corrosive environments as stress corrosion cracking, including failures due to hydrogen embrittlement. However, these two types of cracking respond differently to environmental variables.
Prevention: It is essential to minimize stress levels in materials through stress relief heat treatments. It is also recommended to limit the exposure of components to aggressive corrosive agents, such as chlorides, hydrogen, or sulfur compounds. In addition, the application of protective coatings or surface treatments, such as passivation, helps to form a barrier against corrosive attack.
7. Erosion and cavitation corrosion

Erosion is one of the most aggressive mechanisms of deterioration in hydraulic and industrial equipment, due to the relative movement of a corrosive fluid with respect to the surface of the material. On the other hand, cavitation is a particular type of erosion that occurs when the operating pressure of a fluid falls below its vapor pressure, causing the formation of vapor bubbles that collapse violently on the metal surface.
Prevention: Optimize the hydraulic design to avoid sudden pressure changes and bubble formation in the flow and avoid sudden changes in flow direction. It is advisable to use high-strength materials, such as stainless steels or ceramic coatings, and to apply protective coatings that act as a barrier against the impact of cavitation on exposed surfaces.
8. Desalination (selective leaching): Descaling and graphitization

Selective leaching consists of removing an element from a solid alloy through corrosion processes. The most common example is the selective removal of zinc from brass alloys (de-zincing). Similar processes occur in other alloy systems where aluminum, iron, cobalt, chromium, and other elements are removed.
Prevention: Apply cathodic protection and control the environment, reducing the concentration of dissolved oxygen as much as possible. Another effective measure is the appropriate selection of materials, using resistant alloys such as red brass with 15% zinc, which is immune to this type of attack, or the use of cupronickel in compositions of 70–90% copper and up to 30% nickel, which offer high durability against selective leaching.
Factors influencing metal corrosion
Metal corrosion is a complex electrochemical phenomenon that depends not only on the nature of the material, but also on the environment in which it is found. Among the most influential factors are:
- Metal composition: The presence of alloying elements (such as chromium, molybdenum, or nickel) can increase corrosion resistance, while impurities or non-metallic inclusions often generate anodic sites that promote localized corrosion.
- Environment: The aggressiveness of the environment is a determining factor. Environments with high chloride content, such as seawater or marine atmospheres, increase the likelihood of pitting and cracking. Similarly, relative humidity, temperature, and the presence of gaseous contaminants (SO₂, CO₂, H₂S) modify the kinetics of the corrosive attack.
- Operating conditions: Factors such as flow velocity, turbulence, thermal gradients, and mechanical stresses contribute to accelerating deterioration. For example, in pipes, increased turbulence can remove passive layers and promote erosion corrosion.
- Design and manufacturing: Areas with complex geometries, defective welded joints, or angles that promote fluid accumulation can become critical areas for the initiation of localized corrosion.
The interaction between metallurgical properties and environmental conditions determines the rate and type of corrosion; therefore, understanding these factors is essential for establishing prevention strategies and selecting suitable materials for each industrial application. The following table summarizes these factors:
Table. Most important factors influencing the corrosion of materials
| Factor | Main influence |
|---|---|
| Environment | Humidity, temperature, contaminants, chlorides, and industrial gases |
| Metal properties | Composition, alloying, microstructure, inclusions, and heat treatments |
| Design and operation | Geometry, welds, water accumulation, potential differences, and service conditions |
Predictive monitoring in corrosion management
Predictive corrosion monitoring combines inspection techniques, online sensors, and prediction models to anticipate the deterioration of equipment and infrastructure before a critical failure occurs. Unlike corrective or reactive monitoring, this approach allows for real-time assessment of damage progression and projection of the remaining useful life of assets.
In industry, its application is significant in the integrity of pipelines, process plants, storage systems, and equipment subjected to aggressive environments. The main objective is to detect early patterns of localized or generalized corrosion, integrating field data with prediction algorithms that facilitate decision-making. Among the most widely used techniques are:
- Online electrochemical sensors, such as electrical resistance (ER) and linear polarization (LPR) probes, used to measure the instantaneous corrosion rate.
- Non-destructive testing (NDT) techniques, such as ultrasound, digital radiography, and acoustic emission, to identify thickness loss and defects in the early stages.
- Corrosion models assisted by artificial intelligence and machine learning, used to correlate operating parameters (temperature, pH, chloride concentration, flow velocity) with the probability of future damage
.Predictive monitoring extends asset life, reduces costs from unscheduled shutdowns, improves operational safety, and ensures compliance with international regulations. It also strengthens asset integrity management by integrating corrosion data into broader predictive maintenance systems.
Conclusions
Corrosion is a complex phenomenon that manifests itself in different types of corrosion, ranging from uniform to localized, each with specific mechanisms and consequences for the integrity of materials. Its technical classification allows for a better understanding of the factors involved in the process and the application of the most appropriate control method.
The integration of non-destructive inspection techniques, online sensors, and real-time data analysis allows for more accurate identification of degradation patterns, reducing uncertainty and costs associated with unplanned shutdowns. In this sense, efficient corrosion management depends not only on knowledge of its types and mechanisms, but also on the ability to implement predictive systems that strengthen safety, extend the useful life of assets, and ensure operational continuity in the industry.
References
- Fontana, M. G. (2005). Corrosion Engineering. McGraw-Hill Education.
- Jones, D. A. (1996). Principles and Prevention of Corrosion. Prentice Hall.
- Revie, R. W., & Uhlig, H. H. (2008). Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering. Wiley.
FAQs
What are the most common types of corrosion?
Uniform, localized (pitting and crevice corrosion), galvanic, intergranular, and stress corrosion.
How is corrosion classified technically?
By mechanism (dry or wet), morphology (uniform or localized), and conditions (high temperature, microbial, erosion, cavitation).
What is the difference between uniform and localized corrosion?
Uniform corrosion affects the entire surface; localized corrosion attacks specific areas and is more dangerous.
How is pitting corrosion detected?
Through visual inspection, ultrasound, electrochemical techniques, and scanning electron microscopy (SEM).
What tools are used in predictive monitoring?
Electrochemical probes, fiber optic sensors, automated ultrasound, AI models, and integrity management software.

