Cathodic protection using sacrificial anodes is one of the most effective methods for preventing corrosion in metal structures exposed to aggressive environments, such as marine environments or damp soils. In this technique, sacrificial anodes play a fundamental role. They are designed to corrode in a controlled manner, transferring electrons to the metal to be protected and thus ensuring the integrity of the structure.
The performance of sacrificial anodes depends on their chemical composition, electrochemical potential, the conductivity of the environment, the temperature, and the design of the system. When these factors are correctly balanced, stable, efficient, and long-lasting cathodic protection is achieved, minimizing structural deterioration of materials and extending the useful life of industrial assets.
How sacrificial anodes work
Sacrificial anodes work on the basis of a galvanic cell. When two metals with different electrochemical potentials are connected in a conductive medium (such as seawater), the more active metal (anode) oxidizes, while the more noble metal (protected structure) becomes the cathode, remaining immune to corrosion.
Typical reactions:
Anode: M→M²⁺ + 2e⁻
Cathode: O₂ + 2H₂O + 4e⁻ → 4OH⁻
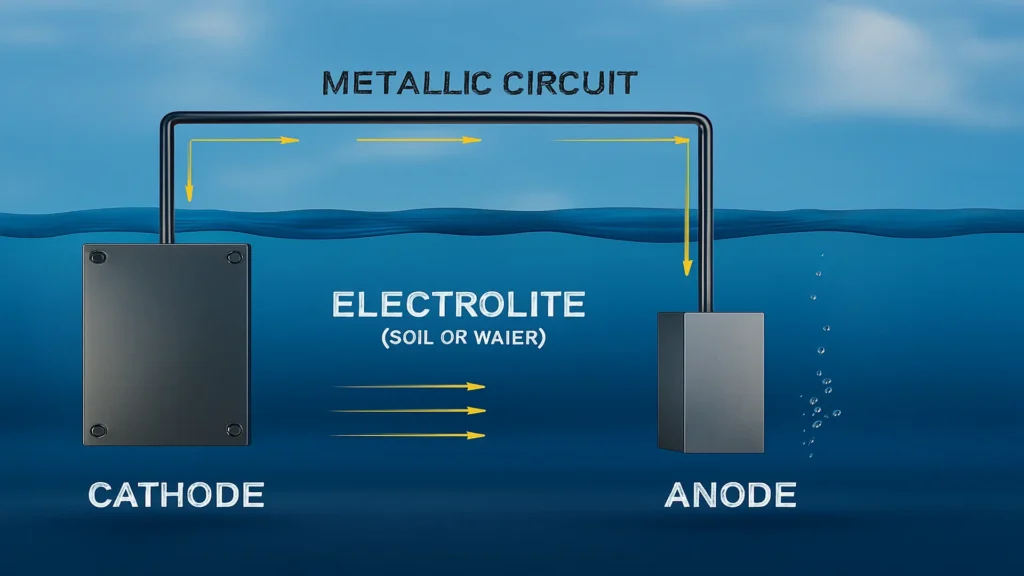
This flow of electrons protects the metal surface of the structure, keeping its potential below the corrosion potential. Therefore, the current efficiency of the anode and its stable working potential are key performance indicators in galvanic cathodic protection systems.
Types of anodes and materials used
The material of the anode determines its electrochemical potential, current density, and service life. The most commonly used are:

🔹 Zinc (Zn) anodes: Recommended in environments with high chloride content and moderate temperatures. Lower current efficiency than aluminum anodes. Typical potential: –1.05 V (vs. Ag/AgCl). High stability in seawater.

🔹 Magnesium anodes (Mg, Mg–Mn–Al) Typical potential: –1.55 V (vs. Cu/CuSO₄). Very active; ideal for soil or fresh water. Shorter life in high conductivity environments.

🔹 Aluminum anodes (Al–Zn–In, Al–Zn–Mg–Ti): Typical potential: –1.10 V (vs. Ag/AgCl). High current efficiency (80–95%). Lower density, which facilitates transport and installation. Possible passivation problems if the composition is not adjusted with indium or gallium.
Table 1. Technical characteristics of the main sacrificial anodes
| Anode Material | Potential (V vs Ag/AgCl) | Efficiency (%) | Typical Application |
|---|---|---|---|
| Zinc | –1.05 | 85–90 | Seawater |
| Al–Zn–In | –1.10 | 90–95 | Offshore structures, platforms |
| Magnesium | –1.55 | 50–60 | Soils, freshwater |
Factors affecting the performance of sacrificial anodes
The performance of sacrificial anodes in cathodic protection systems depends on various factors that act together and determine their efficiency, consumption rate, and the stability of the protection potential achieved. These factors can be classified according to the environment, the material used, the characteristics of the system, and the operating and maintenance conditions.
1.Environmental conditions
The environment in which the cathodic protection system operates has a direct influence on the effectiveness of sacrificial anodes.
- Electrolyte resistivity: This is one of the most relevant parameters in soils with high resistivity or in waters with low conductivity, as the protective current flow is considerably reduced, compromising the effectiveness of the system. The higher the resistivity (for example, in dry soils or waters with low conductivity), the lower the protective current flow.
- Temperature: This variable also modifies electrochemical behavior, as an increase in temperature decreases the resistivity of the medium and accelerates the dissolution of the anode, while low temperatures tend to slow down the process. It affects the dissolution rate of the anode and the resistivity of the medium.
- Chemical composition of the electrolyte: The presence of chlorides, sulfates, or carbonates can alter both the efficiency and consumption rate of the anode.
- Flow and turbulence of the medium: This influences the renewal of the electrolyte around the anode; in conditions of high turbulence, such as in seawater or flow lines, the rate of dissolution and wear of the anode increases, thereby reducing the useful life of the material.
- In seawater or pipes with high flow, dissolution may increase.
2. Properties of the anode material
The properties of the material constitute another set of determining factors.
- Metal composition and purity: Impurities in aluminum, zinc, or magnesium can alter their potential and efficiency.
- Working potential: The composition and purity of the metal directly influence its electrochemical potential and efficiency. Small impurities in aluminum, zinc, or magnesium anodes can alter their behavior and decrease their performance. Each type of material has a characteristic working potential (e.g., Zn ≈ –1.05 V, Al ≈ –1.10 V, and –1.55 V, Mg ≈ –1.55 V vs, referred to an Ag/AgCl electrode), which determines its selection according to the service environment.
- Current density and efficiency: Similarly, current density and electrochemical efficiency (in %) determine what proportion of the material is effectively converted into protective current. The design and geometric shape of the anode are also relevant, as the exposed area directly influences current distribution and the rate of metal consumption.
3. Characteristics of the cathodic protection system
The characteristics of the cathodic protection system are equally decisive.
- Area and type of structure protected: The size, geometry, and type of structure to be protected determine the number and arrangement of anodes required to ensure uniform current distribution. Large or complex surfaces require a greater number or distribution of anodes.
- Electrical connections and continuity: Electrical connections must maintain adequate continuity; faulty connections or high contact resistance drastically reduce the efficiency of the system.
- Protection potential achieved: The protection potential achieved must be maintained within the optimal range, between (–0.80 to –1.10 V vs. Ag/AgCl, depending on the metal), to avoid both underprotection and overprotection of the structure.
- Galvanic interactions: The presence of different metallic materials with different potentials in the same structure can cause galvanic interactions that alter the expected consumption of the anode.
4. Operational and maintenance factors
Operating conditions and maintenance routines have a significant impact on the service life of the cathodic protection system.
- Monitoring and replacement: Regular monitoring of the protection potential allows early deviations to be detected and corrected before failures occur. If the anodes are not replaced in time or if consumption is not properly monitored, the structure may be left unprotected.
- Deposits or fouling: The formation of mineral deposits, biological fouling, or corrosion products on the anodic surface can reduce contact with the electrolyte and, consequently, the available protective current.
- Unforeseen conditions: External factors such as variations in the salinity of the environment, contamination, mechanical damage, or modifications to the structure can alter the expected behavior of the system.
Experimental evaluation and typical results
Anode performance is evaluated through electrochemical and field testing.
The most common tests are:
- Open circuit potential (OCP): measures the initial activity of the anode.
- Polarization curves: determine the operating range and protection potential.
- Current efficiency: percentage of effective current that protects the structure.
- Mass loss: measures the actual rate of anode consumption.
- Post-exposure analysis: inspection using SEM/EDS to study corrosion products.
Representative results:
- Al–Zn–In anodes achieve current efficiencies of 90–95%.
- Zn anodes maintain more stable potentials but with lower efficiency (80–85%).
- Mg anodes, although very active, have a high consumption rate (up to 200 g/A·year).
These results confirm that the selection of the anode must balance potential, consumption, and expected service life.
Performance in various industrial applications
The behavior of sacrificial anodes in different industrial applications reflects the interaction of all the factors previously analyzed: the operating principle, the type of material, the environmental conditions, and the characteristics of the protection system.
1. Marine structures, such as ship hulls, offshore platforms, and submarine pipelines, aluminum and zinc anodes demonstrate high performance due to their stability in seawater and their ability to maintain a potential.
2. Buried systems, such as underground pipes and tanks, the resistivity of the soil and its chemical composition determine the choice of the most suitable material. Magnesium anodes are usually preferred in soils with high resistivity, while in saline or very wet soils it is necessary to adjust the quantity and arrangement of the anodes to prolong their useful life.
3. Industrial process facilities, such as heat exchangers, cooling towers, or condensers, control of the protection potential and the chemistry of the environment is crucial to avoid overprotection or adverse effects on coatings and metal structures. In these environments, zinc anodes offer more stable and predictable performance, maintaining effective protection without compromising the integrity of the assets.
4. Port and coastal storage infrastructure: cycles of exposure to water and air generate differential wear that requires strategic anode design and constant monitoring of the protection potential. Intertidal zones, for example, experience faster consumption due to wetting and drying cycles, highlighting the importance of systematic monitoring and maintenance.
Taken together, this comparative analysis provides an understanding of how different factors interact and how anode performance varies depending on the specific application. Proper material selection, system design, and maintenance planning are important for ensuring effective and long-lasting cathodic protection in any industrial environment, consolidating the knowledge acquired in the previous sections on principles, materials, and factors affecting performance.
Conclusions
The performance of sacrificial anodes is the result of the interaction between material composition, environmental conditions, and system design. Studies show that aluminum alloys modified with indium or gallium offer the best balance of potential, efficiency, and durability in marine environments. However, in low-conductivity soils or waters, magnesium anodes remain the best choice.
Implementing periodic monitoring of protection potential and consumption rate is essential to ensure asset integrity and optimize anode replacement. A well-designed cathodic protection system controls corrosion and transforms industrial maintenance into a preventive, sustainable, and cost-effective practice.
Referencias
- Cottis, R. A. (2017). Shreir’s Corrosion. Elsevier.
- NACE International. (2020). Cathodic Protection Design Guide.
- Oskuie, A., et al. (2021). Evaluación de ánodos sacrificiales a base de aluminio en agua de mar artificial. Corrosion Science, 190, 109736.
- ISO 15589-2:2020. Protección catódica de sistemas de tuberías: tuberías marinas.

