Table of Contents
- What is polylactic acid (PLA)?
- How is PLA produced?
- Mechanical and thermal properties
- How does it differ from conventional plastic?
- Is PLA truly biodegradable?
- Polylactic acid advantages and disadvantages
- PLA in 3D printing: efficiency, quality, and low cost
- The future of PLA: the end of petroleum-based plastic?
- Conclusions
- References
The development of biodegradable polymers has revolutionized the industrial landscape in the search for sustainable alternatives. Among these materials, polylactic acid stands out, a biopolymer derived from renewable sources such as corn starch, sugarcane, or beetroot. Its emergence has sparked growing interest across various industries, as it presents itself as a viable alternative to traditional petroleum-based plastics.
This polymer has positioned itself as a strategic material in sectors seeking to reduce their environmental impact without compromising the functionality of their products. Thanks to its biodegradable and biocompatible nature, it has become an ideal candidate for applications in food packaging, medical devices, 3D printing, and textiles. Its versatility, together with its low carbon impact during production, it at the center of strategies toward a more circular economy.
This article aims to provide a technical and applied review of this material, its properties, production methods, benefits, limitations, and its potential role in the transition to more sustainable materials.
What is polylactic acid (PLA)?
Also known as polylactic acid, it is an aliphatic, biodegradable thermoplastic polymer produced from lactic acid obtained by fermenting sugars. There is currently growing interest in the use of biodegradable polymers as a substitute for petrochemical-based polymers.1.
Molecular chemical structure
Polylactic acid is a linear polymer consisting of repeating monomeric units of lactic acid. Each of these units contains an ester group (–COO–) formed by the condensation reaction between the carboxyl group (–COOH) of one lactic acid molecule and the hydroxyl group (–OH) of another. The result is a chain of ester bonds that repeats throughout the polymer (Figure 1).
The general structural formula can be represented as (–O–CH(CH₃)–CO–)ₙ, where “n” indicates the number of repeating units in the polymer chain. This structure includes a methyl group (–CH₃) attached to a chiral carbon, allowing for the existence of different stereoisomeric forms such as PLLA (levorotatory), PDLA (dextrorotatory), or PDLLA (racemic).
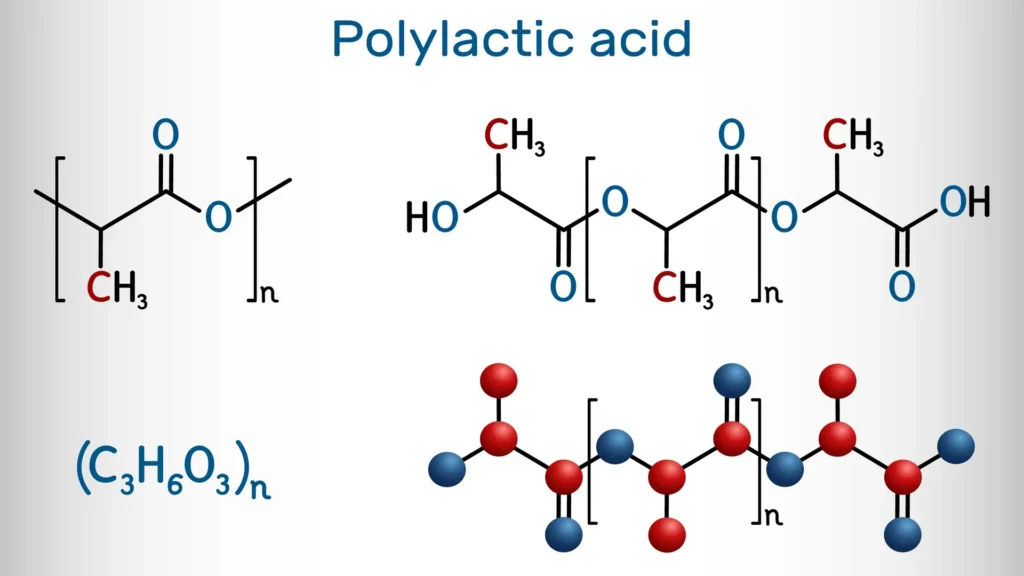
Each monomeric unit contributes a three-carbon skeleton, one of which forms part of the ester group, another carries the methyl group, and the third connects the main chain. Therefore, it can also be described as consisting of the repeating unit –O–C₃H₄O₂–, or more briefly as (O–C₃CO)ₙ, highlighting the carbon-oxygen skeleton. The structure can be linear or, in some cases, slightly branched, depending on the synthesis process.
How is PLA produced?
Polylactic acid is obtained from lactic acid (base monomer), which is a natural organic compound produced by chemical synthesis or, preferably, by fermentation of carbohydrates from renewable raw materials. The most common substrates include corn starch, sugarcane juice, cassava, and agricultural waste2.
During fermentation, microorganisms such as Lactobacillus metabolize sugars to produce lactic acid, a colorless liquid that can occur in optically active forms (L-lactic acid, D-lactic acid, and racemic). The form used influences the properties of the final polymer, such as its crystallinity, thermal resistance, and biodegradability.
Lactic acid has good solubility properties in water and alcohol, but is insoluble in solvents such as chloroform or carbon disulfide. Its purification is important for producing high-quality lactic acid, and one of the technological challenges is to reduce the costs associated with this process, especially on an industrial scale3.
Polymerization methods for production
There are two main methods for transforming lactic acid into polylactic acid:
Direct polycondensation polymerization
This method involves the direct reaction of lactic acid with the release of water as a by-product. Although it is a simple process, it has limitations in controlling the molecular weight of the polymer due to difficulties in removing residual water during the final stage of the reaction. This leads to lower quality polymers, which may not be suitable for demanding applications.
Ring-opening polymerization of lactic acid. ROP
This is the most efficient and commercially used method. It starts with a cyclic dimer called lactide, which is obtained by controlled depolymerization of lactic acid. Then, through a catalyzed process, the ring is opened and long polymer chains are formed. This method allows for high molecular weights and better structural homogeneity, ideal for advanced industrial applications4.
The process consists of three stages: polycondensation (R1), depolymerization (R2), and ring-opening polymerization (R3) (Figure 1).
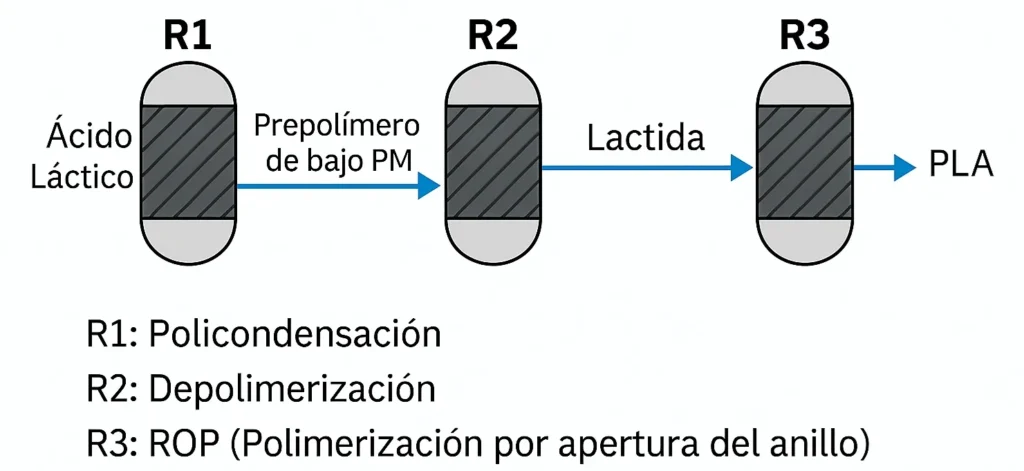
This route requires additional purification steps that are relatively complicated and costly. One advantage of this route is the possibility of controlling the configuration of the monomer units, adjusting the thermal and mechanical properties of the resulting polymer5. However, the synthesis of lactic acid and its purification involve additional costs and technical complexities.
Mechanical and thermal properties
Table 1. Mechanical and thermal properties
| Category | Property / characteristic | Detail |
| Mechanical properties | Tensile strength | ~3309 MPa |
| Yield strength | 55 MPa | |
| Flexural strength | 485 MPa | |
| Compressive strength | 66 MPa | |
| Elongation at break | ~3% (low) | |
| Brittleness | High; easily breaks under stress or impact | |
| Thermal properties | Heat distortion temperature | 50–60 °C |
| Melting point | 170–180 °C | |
| Heat resistance | Low; not suitable for high temperatures |
How does it differ from conventional plastic?
In general, when analyzing the differences between PLA and PET or other petrochemical compounds, it is difficult to argue that they are better than their more conventional counterparts. In fact, there is growing evidence to suggest that they may be more harmful to the environment than standard products.
Although renewable raw materials are used in their manufacture, these come at an additional cost, as they not only require significant use of non-renewable resources, but also have a negative impact on the environment in several ways. Furthermore, although this compound can mitigate the growing problem of microplastics, it can hardly be classified as compostable with existing infrastructure.
However, these negative aspects fuel a larger problem, namely the perpetuation of single-use products and a shift away from the narrative of reduction and reuse. The truth is that any single-use product is far from being an ideal solution to our current waste generation problems, and the priority should be to reduce consumption or switch to reusable packaging.
Is PLA truly biodegradable?
Yes, polylactic acid is a biodegradable material, but it requires specific conditions and does not occur naturally in all environments. Contrary to what is sometimes suggested, it does not decompose quickly in the environment.
- Biodegradable under specific conditions: It degrades under industrial composting conditions, where high temperatures are reached, but not necessarily in home composting or natural environments.
- Environmental conditions: In natural environments, biodegradation is slower and less efficient than under industrial composting conditions.
- Industrial vs. domestic composting: It degrades effectively in industrial composting facilities, but is not recommended for domestic composting due to the lack of necessary conditions (temperature, humidity, etc.).
- Contamination risk: If disposed of incorrectly in the environment, it can contribute to contamination, as its decomposition is very slow in the absence of favorable conditions.
- Recycling: It is recyclable, which may be a more sustainable option for its management after use.
- Considerations regarding origin: It is produced from renewable resources such as corn, but the question has also been raised whether its large-scale production is justified considering the use of food as raw material.
Although this polymer is biodegradable, the process requires specific conditions and does not occur naturally in all environments. It is important to consider its proper management, whether through recycling or industrial composting, to prevent environmental pollution.
Polylactic acid advantages and disadvantages
What advantages does it offer over petroleum-based polymers?
It reduces CO2 emissions: In the fight to mitigate the consequences of climate change, polylactic acid represents a favorable alternative, as it generates fewer greenhouse gas emissions compared to conventional. For example, manufacturing one kilogram of plastic from scratch emits 3.5 kg of CO2, whereas manufacturing the same amount from recyclable material emits only 1.7 kg of CO2.
Promotes sustainability: One of the advantages of this material is that, as it is derived from renewable sources, it is a much more environmentally friendly option than other sources, such as petroleum derivatives.
It can be applied to a variety of products: Depending on the industry, polylactic acid can have endless applications. Although this article has focused on its use in the manufacture of food chain products, it is also useful in medical products, skin care products, 3D printing, among others.
It is safe for living beings: Is biocompatible, which means that it can come into contact with living organisms without posing a health risk. In fact, it is certified worldwide by regulatory agencies for contact with food.
Technical limitations (temperature, degradation)
Like any innovation, this material faces challenges. Its thermal behavior, the need for specific conditions for biodegradation, and competition with food crops as a raw material are aspects that must be evaluated critically.
Table 2. Technical Limitations and Degradation
| Category | Limitation / Characteristic | Detail |
| Technical limitations | Brittleness | Limited in applications involving stress or impact |
| Low heat resistance | Easily deforms at moderate temperatures | |
| Impact resistance | Low compared to other plastics like ABS | |
| Degradation | Compostability | Degrades under industrial composting conditions |
| Natural biodegradation | Slow and inefficient | |
| Factors accelerating degradation | High temperature, humidity, and UV light exposure |
PLA in 3D printing: efficiency, quality, and low cost
Polylactic acid has established itself as one of the most widely used materials in the world of 3D printing, especially in FDM (Fused Deposition Modeling) technology. Its popularity is due to a combination of factors that make it attractive to both novice users and professionals in design and additive manufacturing.
- Ease of printing: One of the main advantages over other thermoplastics is its ease of use. It does not require a mandatory heated bed or a closed printer, which reduces the complexity of the process and makes it possible to print even with home-level equipment. Its extrusion temperature ranges from 190°C to 220°C, which also allows it to be used with standard nozzles without the risk of premature deterioration. In addition, does not give off strong odors or toxic fumes during the printing process, making it ideal for educational environments, offices, or workshops without specialized ventilation.
- High-quality finishes: Another notable aspect is its ability to produce smooth, well-defined surfaces, which is essential for visual prototypes, architectural models, or decorative pieces. The filament’s good performance during extrusion reduces stringing and improves adhesion between layers, producing clean prints that are highly faithful to the original design.
- Competitive cost and accessibility: Stands out as one of the most economical and accessible filaments on the 3D printing market. Its relatively low cost compared to other materials makes it an attractive option for users of all levels, from beginners to professionals. Even modified versions such as PLA+, which offer improved mechanical properties, maintain a favorable cost-benefit ratio. While prices may vary between manufacturers and suppliers, PLA remains one of the most competitive materials in terms of affordability and performance.
- Ideal for multiple applications: Due to its good properties, is used in a wide variety of applications: from rapid prototyping and production of low-stress functional parts to school projects, decorative parts, and toys. Its ease of use, combined with good thermal and dimensional behavior, makes it a favorite choice in the 3D printing ecosystem.
The following video provides an overview of PLA filament, expound its origin, properties, and how it works with MakerBot 3D printers. Source: Proto3000

3D printing of filaments with PLA..
The future of PLA: the end of petroleum-based plastic?
Looking ahead, in a global scenario marked by the urgent need to reduce dependence on fossil fuels and mitigate the environmental impacts of traditional , polylactic acid is emerging as a disruptive alternative.
Derived from renewable sources such as corn starch, sugarcane, or beetroot, it offers a sustainable alternative to petroleum-based polymers: it embodies a transition toward more sustainable materials designed to reduce the environmental footprint of modern industry.
Its ability to biodegrade under industrial conditions, coupled with its low carbon impact during production, positions it as a strategic material in the progressive substitute of conventional plastics.
Although its technical limitations, such as low thermal and mechanical resistance compared to conventional polymers, restrict its use in certain demanding applications, the continuous improvement of its formulations, such as PLA+, and its growing adoption in sectors such as packaging, 3D printing, and medicine, herald a new paradigm. It challenges petroleum-based; it may be the beginning of a new era of materials designed not only to function, but also to coexist with the planet.
Conclusions
PLA is currently one of the most researched and widely used biodegradable polymers due to its renewable origin, versatility, and potential to reduce environmental impact. Although it has not yet completely alternate petroleum-based plastic, its constant evolution and growing adoption position it as one of the pillars of the transition to more responsible materials.
To maximize its potential, it will be key to continue improving its properties, reducing its production costs, and developing adequate recycling and composting infrastructures. But above all, it will be necessary to rethink the use of materials in a model that prioritizes efficiency, reduction, and reuse. The future of polylactic acid does not depend solely on its performance as a material, but on the collective drive to transform the way we consume, produce, and interact with the environment.
References
- A. Samrot; T. C. Sean; T. Kudaiyappan; U. Bisyarah; A. Mirarmandi; E. Faradjeva; A. Abubakar; H.H. Ali; J.L.A. Angalene y S. Kumar. «Producción, caracterización y aplicación de nanotransportadores fabricados con polisacáridos, proteínas, biopoliésteres y otros biopolímeros: una revisión», International Journal of Biological Macromolecules, vol. 165, n.º B15, pp. 3088-3105, 2020. DOI: https://doi.org/10.1016/j.ijbiomac.2020.10.104
- G. Luna; H. Villada y R. Velasco. «Almidón termoplástico de yuca reforzado con fibra de fique: preliminares», DYNA, vol. 76, n.º 159, pp. 145-151, 2009. http://www.scielo.org.co/pdf/dyna/v76n159/a15v76n159.pd
- B. Wojtyniak; J. Kołodziejczyk y D. Szaniawska. «Producción de ácido láctico mediante ultrafiltración de suero fermentado obtenido en un biorreactor equipado con membrana ZOSS», Chemical Engineering Journal, vol. 305, n.º 1, pp. 28-36, 2016. DOI: https://doi.org/10.1016/j.cej.2016.01.048
- A. A. Siles. Síntesis, propiedades y aplicación del ácido poliláctico a partir del almidón de la papa. Trabajo de grado para optar al título de Ingeniero de Materiales. Universidad Nacional de San Agustín. Perú, 2014. Disponible: http://repositorio.unsa.edu.pe/handle/UNSA/2885
- M. Nofar; D. Sacligil; P. J. Carreau; M. R. Kamal and M. C. Heuzey. “Poly (lactic acid) blends: Processing, properties and applications”, International Journal of Biological Macromolecules, vol. 125, 307–360, 2019. DOI: https://doi.org/10.1016/j.ijbiomac.2018.12.002