Among the different types of corrosive damage, crevice corrosion represents one of the most problematic mechanisms. Unlike uniform corrosion, which is visible and predictable, this phenomenon appears in localized areas such as flanges, gaskets, lap joints, and bolted connections, where design and environment are decisive in creating highly aggressive electrochemical conditions.
Considered a critical form of localized corrosion, it has been responsible for premature failures in carbon steels, stainless steels, and advanced alloys, even in systems that met design and operating specifications. Its danger lies not only in material loss, but also in its ability to progress without evident external signs, compromising the integrity of metallic structures in industry.
Crevice corrosion: Concept and mechanism
Generally, it develops when an electrolyte becomes trapped in confined spaces, generating a highly aggressive environment for metallic surfaces. This localized corrosion mechanism can occur both between two metals in contact and between a metal and a non-metallic material, such as gaskets or seals. Its action is concentrated in small areas of larger components, establishing itself in microenvironments formed by joints, overlaps, or geometric discontinuities. It frequently occurs in riveted joints, lap joints, oxide deposits, among others. The following image shows an example of this type of corrosion.
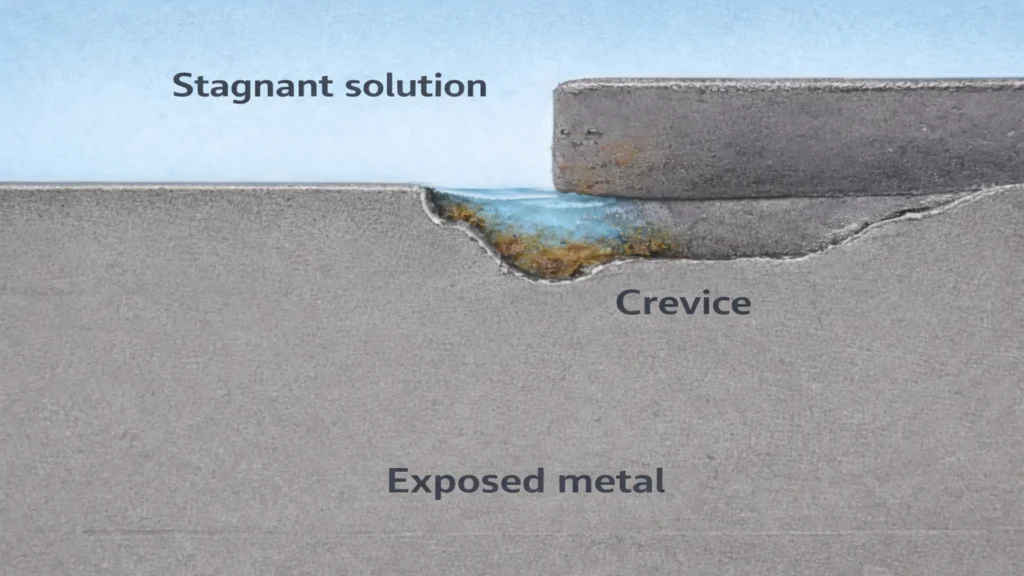
Due to its hidden nature, it often goes unnoticed for long periods. The combination of oxygen depletion and the progressive acidification of the medium inside the crevice creates ideal electrochemical conditions for accelerated propagation.
This type of corrosion initiates when a geometric discontinuity limits the exchange of the electrolyte with the external environment. These crevices may be generated by design (flanges, gaskets), poor assembly, or surface deposits.
Electrochemical mechanism of crevice corrosion
From an electrochemical standpoint, the process develops in several well-defined stages:
- Oxygen depletion: Inside the crevice, oxygen is rapidly consumed and cannot be replenished, creating an oxygen concentration cell.
- Formation of differential cells: The exterior of the crevice becomes the cathode, while the interior acts as an active anode, accelerating metal dissolution.
- Acidification of the microenvironment: The hydrolysis of metal ions generates a local pH drop, intensifying environmental aggressiveness.
- Chloride migration: To maintain electroneutrality, chloride ions migrate into the crevice, breaking passive layers and promoting localized corrosion.
In summary, observing the following image, the electrochemical mechanism occurs in two phases.
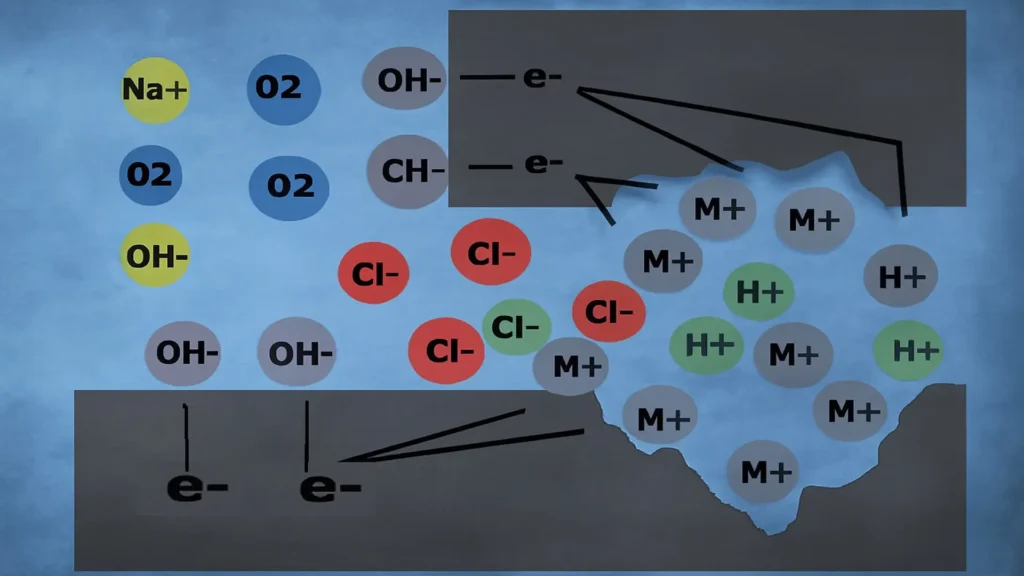
First phase:
- Metal oxidation: M → M⁺ + e⁻
- Oxygen reduction: O₂ + 2H₂O + 4e⁻ → 4OH⁻
Second phase:
- Autocatalytic process: M + Cl⁻ + H₂O → MOH⁻ + H⁺Cl⁻
Due to oxygen depletion in the confined space, the oxygen reduction reaction does not occur; instead, an autocatalytic reaction takes place. The hydrogen ion reacts with the chloride ion from seawater, forming hydrochloric acid, producing an attack on the metallic material in the form of cracks or crevices.
This mechanism explains why cracks can advance even in materials considered resistant, overcoming passive barriers and accelerating section loss.
Causes, detection, and control
Main causes
- Confined and poorly drained geometries.
- Presence of flanges, porous or poorly compressed gaskets.
- Chloride-containing environments (marine, industrial, chemical).
- Poor material selection.
- Lack of maintenance or targeted inspection.
Detection: The major challenge
One of the greatest risks is its hidden nature. Often, damage is only discovered after disassembly, leaks, or mechanical failures. Techniques such as:
- Targeted visual inspection.
- High-resolution ultrasonic testing.
- Advanced NDT (eddy currents, digital radiography) is essential to identify this type of damage.
Control strategies
- Design that eliminates unnecessary crevices.
- Use of non-absorbent seals and appropriate gaskets.
- Properly designed cathodic protection.
- Application of continuous coatings.
- Risk-Based Inspection (RBI) programs.
The approach promoted by organizations such as AMPP emphasizes prevention from the design stage and integrated corrosion management.
Materials susceptible to crevice corrosion
Susceptibility varies significantly depending on the material:
- Carbon steels: Highly vulnerable in humid or chloride-containing environments, especially under deposits.
- Stainless steels: Although passive, they may fail rapidly if the protective film breaks down in confined areas.
- Nickel and titanium alloys: Greater resistance, but not immune under extreme conditions.
Material selection must consider not only chemical composition, but also system design and the actual operating environment.
Why is crevice corrosion dangerous?
It is dangerous because it is not easily visible, does not produce extensive oxides, does not change the color of large surfaces, and does not generate immediate visible losses. However, its localized progression can cause:
- Sudden perforations.
- Unexpected leaks.
- Structural failures without warning.
- High costs due to unplanned shutdowns.
In most cases, when damage becomes visible, the component’s integrity is already compromised.
Conclusions
Crevice corrosion constitutes a critical localized corrosion mechanism, as it can develop even in materials and systems that meet design specifications, advancing silently and in a highly aggressive manner.
The combination of unfavorable geometric design and stagnant environments promotes the formation of electrochemical gradients within the crevice, accelerating the degradation of carbon steels, stainless steels, and structural alloys.
Effective prevention of crevice corrosion requires an integrated approach, including appropriate design criteria, correct material selection, environmental control, and early inspection programs focused on localized damage.
References
- Fontana, M. G. (1986). Corrosion engineering (3rd ed.). McGraw-Hill.
- Revie, R. W., & Uhlig, H. H. (2008). Corrosion and corrosion control: An introduction to corrosion science and engineering (4th ed.). John Wiley & Sons.
- Jones, D. A. (1996). Principles and prevention of corrosion (2nd ed.). Prentice Hall.
Frequently Asked Questions (FAQs)
What is localized corrosion and why is it dangerous?
Localized corrosion is a type of attack concentrated in specific areas of the material. It is dangerous because it can cause rapid failures with minimal total mass loss, making early detection difficult.
Why does corrosion occur in flanges and bolted joints?
Flanges and bolted joints create natural crevices where moisture accumulates, oxygen is limited, and differential electrochemical cells are promoted—ideal conditions for crevice corrosion.
How can hidden corrosion in industrial equipment be detected?
Through targeted inspections, NDT techniques, thickness monitoring, and scheduled disassembly in critical areas, especially in equipment exposed to aggressive environments.

