Table of Contents
Fractional distillation is one of the most important separation processes in the process industry and, particularly, in refinery distillation for the separation of hydrocarbons derived from crude oil. Its relevance lies in its ability to obtain high-purity products, operate on a large scale, and adapt to both ideal and non-ideal mixtures. However, current requirements for quality, energy efficiency, and environmental impact reduction demand that the fractionating column operate under optimal conditions, making the use of advanced control strategies and a thorough analysis of process stability indispensable.
What is fractional distillation and how does it work?
Fractional distillation is a unit operation that separates a liquid mixture into different fractions by taking advantage of differences in boiling points or relative volatilities of its components. This process is carried out in a distillation column equipped with trays or packing, a reboiler at the bottom, and a condenser at the top. In the rectifying section, the vapor becomes enriched in the more volatile component, while in the stripping section these components are removed from the liquid through the addition of heat.
In crude oil fractional distillation, the column allows the progressive separation of fractions such as gases, naphtha, kerosene, and gas oils, constituting the core of the fractional distillation configuration in a refinery.
Parts of a column
A fractionation column is an essential piece of equipment in separation processes based on volatility differences. Its main components are:
- Column (or Tower): Vertical structure where separation occurs. It contains trays or packing distributed along its height.
- Internal trays: Perforated horizontal surfaces that allow contact between rising vapor and descending liquid, promoting phase equilibrium.
- Feed: Entry of the mixture to be separated, strategically located at an intermediate tray according to composition.
- Reboiler: Heat exchanger at the base that vaporizes the bottom liquid, generating the vapor that rises through the column.
- Condenser: Located at the top, it cools the vapor leaving the column, converting it into liquid.
- Reflux drum (Reflux accumulator): Vessel that receives the condensate; part is returned to the column as reflux, and part is withdrawn as distillate product.
- Bottom product: Less volatile fraction withdrawn from the bottom of the column.
- Distillate product: More volatile fraction collected after condensation.
The following image shows the process diagram of the tower and each of its components:
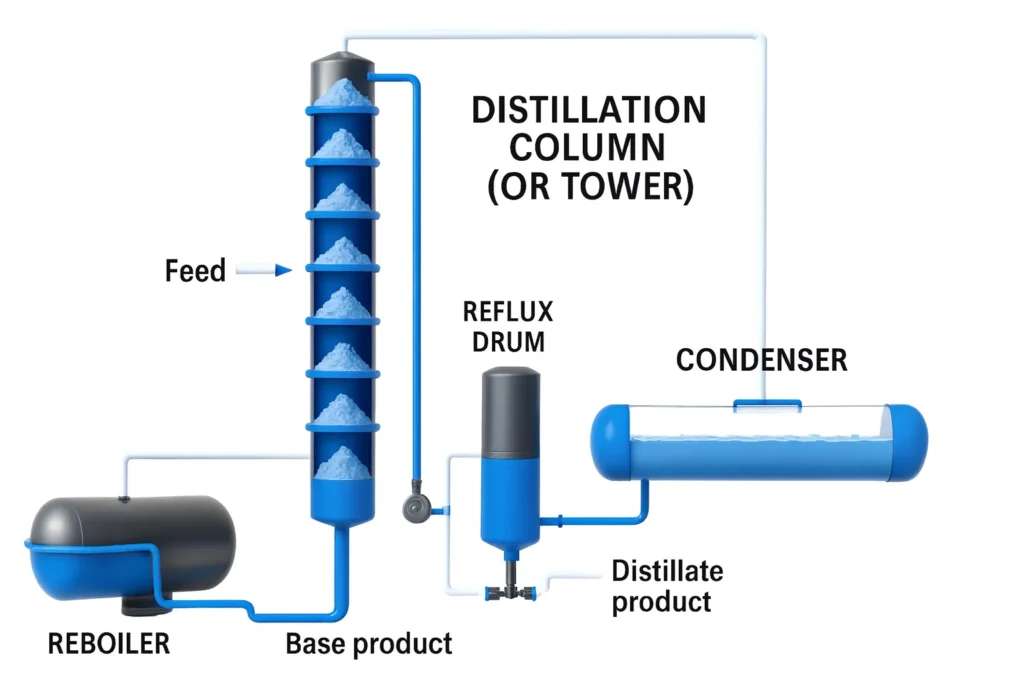
Operation of the Fractionation Column
The fractionation process is based on continuous distillation, where components of a liquid mixture are separated according to their boiling points:
- Feed entry: The mixture enters an intermediate tray. More volatile components tend to rise, while less volatile ones move downward.
- Vapor generation: The reboiler heats the bottom liquid, generating vapor that rises through the column.
- Tray interaction: On each tray, rising vapor and descending liquid interact, allowing mass and energy transfer. This enhances stage-wise separation.
- Top condensation: Vapor reaching the top is condensed in the condenser.
- Reflux: Part of the condensate is returned to the column to enrich separation; the remainder is withdrawn as distillate product.
- Product withdrawal: The more volatile product is collected at the top (distillate), and the less volatile product is withdrawn at the bottom (bottoms product).
This dynamic equilibrium allows the production of high-purity products by adjusting variables such as temperature, pressure, number of trays, and reflux ratio.
Types of fractional distillation
There are several types of fractional distillation depending on the nature of the mixture and operating conditions. Among the most relevant are continuous fractional distillation, widely used in refineries; batch distillation, common in the pharmaceutical industry; and vacuum distillation, applied when components have high boiling points. Each type presents specific challenges in terms of control and dynamic stability.
The types of fractional distillation are described below:
- Continuous fractional distillation: This is the most widely used type in the process industry. It operates continuously with constant feed and product withdrawal, allowing high production capacities and stable operation, and is characteristic of petroleum refineries and petrochemical plants.
- Batch fractional distillation: This process is carried out by charging a defined quantity of mixture into the system and processing it in cycles. It offers high operational flexibility but lower productivity, and is therefore mainly used in the pharmaceutical and fine chemical industries.
- Vacuum fractional distillation: Operates at reduced pressures to lower the boiling points of components. This method prevents thermal degradation of heat-sensitive substances and is essential in the separation of heavy crude oil fractions.
- High-pressure fractional distillation: Conducted at pressures above atmospheric to facilitate the condensation of highly volatile components. It is applied in the separation of liquefied gases and processes requiring greater control of vapor–liquid equilibrium.
- Azeotropic fractional distillation: Used when a mixture forms an azeotrope that prevents conventional separation. By adding an external agent, the system equilibrium is modified and the desired separation is achieved.
- Extractive fractional distillation: Uses a high-boiling solvent that alters the relative volatility of components without forming azeotropes. It is common in separating mixtures with very close boiling points, especially in the petrochemical industry.
- Reactive fractional distillation: Integrates a chemical reaction and distillation separation simultaneously within the same column. This approach improves reactant conversion and process efficiency but involves greater complexity in control and operation.
Control in fractional distillation columns
The Fractionating Column as a Dynamic System
From a process control perspective, the fractionating column is a highly interactive dynamic system, where variables such as pressure, temperature, levels, and composition are strongly coupled. Liquid holdup, the type of internals (trays or packing), and residence times directly influence the system’s dynamic response and stability.
Control in distillation columns is essential due to their highly nonlinear behavior and strong variable interactions. A modern automation system integrates basic regulatory control with advanced process control (APC) strategies, enabling faster responses to disturbances and improved operational stability.
Advanced control and process stability
Advanced control in fractional distillation, supported by Advanced Process Control (APC) techniques, allows anticipation of disturbances, minimization of product quality variability, and optimization of energy consumption. Process stability is achieved through an appropriate control philosophy that combines classical regulatory loops with advanced real-time supervision and optimization strategies.
The fundamental variables to be controlled include:
- Pressure, which directly affects vapor–liquid equilibrium.
- Level, to ensure material balance.
- Temperature, as an indirect composition variable.
- Composition, to ensure the quality of distillate and bottoms products.
The image illustrates the fundamental control strategies in a distillation column, highlighting the four main types: pressure, level, composition, and temperature control. These variables are essential to ensure stable, efficient, and safe operation of the separation process.
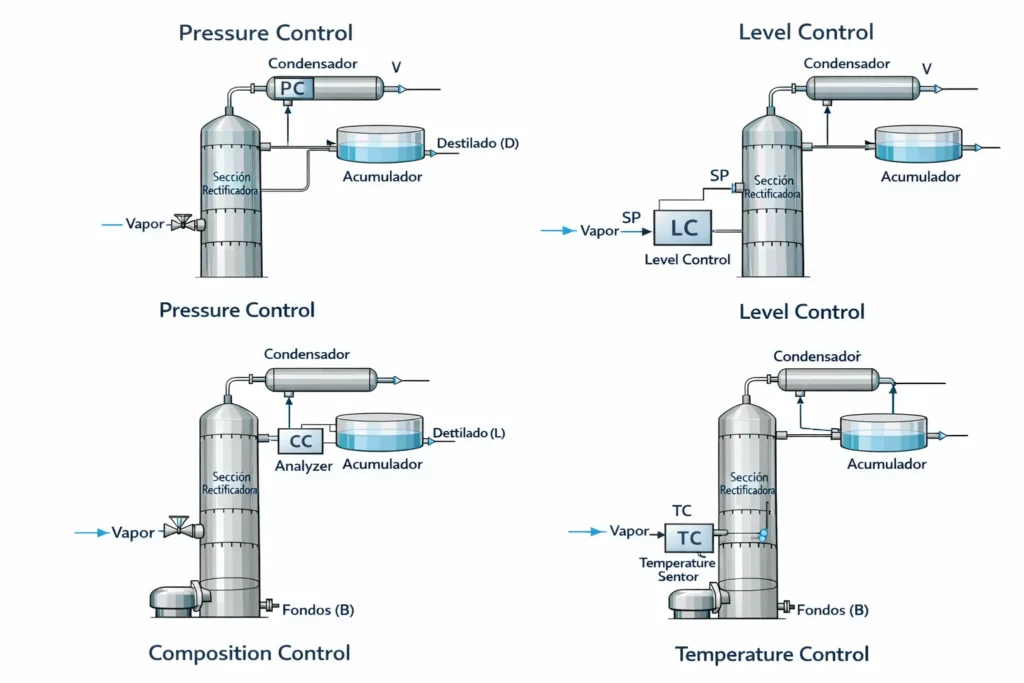
Pressure control
Pressure within the column is a critical variable, as it directly influences condensation, vaporization, temperatures, and product compositions. Precise pressure control prevents excessive vapor accumulation and maintains energy balance. For example, if the supplied heat exceeds the removed heat, excess vapor is generated and pressure increases. In practice, when a total condenser is available, pressure is regulated by manipulating the condenser cooling water flow, pairing the controlled variable (CV) with the manipulated variable (MV) to achieve stability.
Level control
The level in the overhead and bottom accumulators ensures material balance within the column. In the overhead accumulator, level is usually controlled using the reflux flow, especially when the reflux-to-distillate ratio is high, since distillate flow has less influence. Bottom level is typically regulated by the bottoms withdrawal flow, unless the bottom volume is small, in which case reboiler duty may be used, although this practice is uncommon due to the phenomenon of “inverse response.”
Composition control
Ideally, both distillate and bottoms compositions are controlled to maintain product quality specifications, a strategy known as dual end composition control. However, in practice, controlling both variables simultaneously creates interactions that may destabilize the column. To avoid this, single end composition control is implemented, regulating the composition of the most critical variable while allowing the other to vary through simple or detuned flow control.
Temperature control
Temperature is an indirect but fundamental control variable, especially at strategic points in the column where temperature profiles are monitored. Using temperature sensors and controllers, heat input or reflux is adjusted to maintain stable and reproducible conditions, ensuring the desired separation and preventing deviations in composition or energy efficiency.
Together, these strategies enable integrated control of the distillation column, ensuring stability, safety, and compliance with process specifications, as illustrated in the image.
Why is advanced control important in fractionation Columns?
Advanced control in fractionation columns is essential to maximize operational, energy, and economic efficiency in the fractional distillation process. These columns represent one of the highest energy consumers in an industrial plant, especially in refineries and petrochemical complexes, where the reboiler and condensation systems demand large amounts of steam and cooling services. Conventional control is insufficient to optimally respond to the complex process dynamics and frequent feed disturbances.
From an energy efficiency standpoint, advanced control allows the column to operate near its optimal point by coordinating key variables such as reflux, reboiler duty, pressure, and temperature profiles. Through Advanced Process Control (APC) techniques, excessive energy use can be reduced without sacrificing product quality, avoiding unnecessary safety margins that increase energy consumption.
Additionally, advanced control improves separation efficiency by minimizing variability in product composition. By maintaining tighter specifications, the need for over-purification is reduced, directly resulting in lower thermal loads and better utilization of the column’s installed capacity. This allows increased process throughput without additional equipment investment.
Another key aspect is the ability of advanced control to anticipate disturbances, such as changes in feed composition or operating conditions. By acting predictively, the system avoids prolonged deviations that typically generate energy losses and off-spec products. In this way, process stability is improved and resource utilization is optimized.
Overall, implementing advanced control in fractionation columns not only ensures safer and more stable operation, but also represents a strategic tool to improve energy efficiency, reduce operating costs, and move toward more sustainable and competitive processes.
Fractional distillation and energy efficiency are closely related, as this operation is energy-intensive. Proper control of reflux, reboiler duty, and temperature profiles allows operating costs to be reduced without compromising separation performance.
Conclusions
Fractional distillation remains a key operation in the separation of hydrocarbons and other multicomponent systems. The implementation of advanced control strategies not only improves process stability, but also increases energy efficiency and ensures compliance with quality specifications, consolidating the distillation column as a strategic element within the process industry.
Each type of fractional distillation responds to specific process needs. Proper selection depends on factors such as mixture nature, operating conditions, energy efficiency, and control complexity, with continuous distillation being the most representative in refineries and large industrial processes.
References
- Treybal, R. E. (1988). Mass-transfer operations (3rd ed.). McGraw-Hill.
- Foust, A. S., Wenzel, L. A., Clump, C. W., Maus, L., & Andersen, L. B. (1980). Principles of unit operations (2nd ed.). CECSA.
- McCabe, W. L., Smith, J. C., & Harriott, P. (2005). Unit operations of chemical engineering (7th ed.). McGraw-Hill.

