Table of Contents
- What is SCC, and why does it affect 316 stainless steel?
- Metallurgical mechanism behind stress corrosion
- Factors contributing to SCC in 316 steel welds
- Manifestation of SCC in stainless steel welds
- Recent advances in research
- Prevention of SCC in 316 stainless steel welds
- Conclusions
- References
- FAQs about SCC on welded 316 stainless steel
316 stainless steel is widely used for its corrosion resistance and good toughness, but it can be seriously compromised by Stress Corrosion Cracking in 316 stainless steel welds (SCC), especially in welded areas. This phenomenon occurs when mechanical stresses (such as residual stresses generated during welding), chloride-rich environments and elevated temperatures are combined.
Welded joints are particularly vulnerable due to their heterogeneous microstructure and the presence of stress concentrators. These conditions favor SCC crack initiation, which can lead to accelerated degradation in welded components if adequate preventive measures are not implemented.
What is SCC, and why does it affect 316 stainless steel?
Stress corrosion cracking (SCC), is a degradation phenomenon that combines the simultaneous action of a corrosive environment, residual or applied stresses, and elevated temperatures. In the case of 316 stainless steel, a material widely used for its general corrosion resistance and mechanical stability, this type of corrosion can occur when in chloride environments, especially if there are micro-cracks and internal stresses in the welded areas.
The vulnerability of 316 in the presence of chlorides and stresses is due to its composition, which, although resistant to chloride-induced pitting and crevice corrosion, is not completely immune to SCC. The combination of mechanical loading and chemical attack can initiate an corrosion process in welded components, compromising the structural integrity of critical equipment.
Metallurgical mechanism behind stress corrosion
Welded joints of austenitic stainless steels such as 316 are particularly susceptible to SCC due to their microstructural heterogeneity, residual stresses and stress concentrators. Thermal sensitization, with formation of chromium carbides at grain boundaries, reduces the corrosion resistance of the material1. The associated metallurgical mechanism generated by these processes is discussed below.
Sensitization and chromium carbide formation
Stainless steels base their resistance to corrosion in oxidizing environments on the presence of chromium in solution (dissolved) in the gamma iron (austenite) or ferrite phase.
When welding 316 stainless steel, especially under conditions where thermal control is not optimal, a critical metallurgical phenomenon known as sensitization can occur, Figure 1. This process occurs in the heat-affected zone, where welding temperatures (approximately 450 °C to 850 °C) allow chromium carbides (Cr₃C₆) to form at the grain boundaries of the steel.
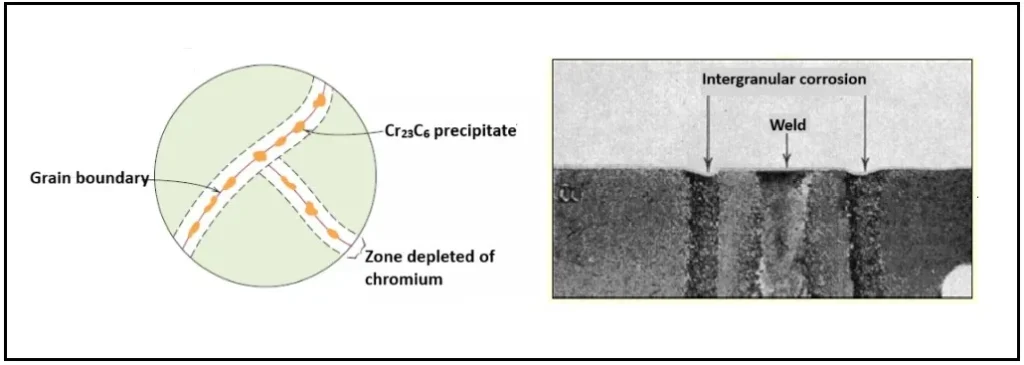
Figure 1. Conditions that promote sensitization of stainless steels:
- %C contents > 0.03 and no stabilizing elements (Ti, Nb)
- Prolonged exposures to temperatures between 450°C and 870°C (welding).
Formation of chromium carbides: From a thermodynamic perspective, the formation of these carbides is favorable: chromium has an affinity for the carbon present in the alloy, and the heat of welding provides sufficient energy for these compounds to precipitate. The problem is that, as the carbides form at the grain boundaries, a local depletion of chromium is generated in the adjacent matrix, preventing the formation of the passive chromium oxide film that protects the stainless steel against corrosion.
Loss of strength in the welded zone
Loss of strength in the welded zone: This chromium depletion makes the microstructure vulnerable to intergranular corrosion, and when welding residual stresses and chloride environments are combined, the risk of stress corrosion cracking increases significantly. The sensitized zone not only loses corrosion resistance, but also exhibits lower mechanical strength compared to the unwelded base material.
The microstructure in the weld heat-affected zone is coarse and carbides form at the grain boundaries, which reduces strength. The precipitation of carbides at the grain boundaries reduces the Cr content in the crystal, which results in a decrease of the corrosion resistance of the passivation film on the stainless steel leading to crack formation, as shown in the figure 2.
The weld may have lower strength precisely because of this carbide formation, making the welded area a weak point. To solve this problem, it is recommended to use low carbon stainless steels (such as 316L), which reduces the formation of carbides and, therefore, sensitization. It is also recommended to follow strict preheating and controlled cooling procedures to limit the time the material remains in the critical temperature range.
In summary, the combination of mechanical loading, chemical attack by chlorides and thermally induced metallurgical transformations lead to SCC in 316 stainless steel welds. Therefore, understanding and controlling these mechanisms is as important as monitoring the environment and applying good design and maintenance practices.
Factors contributing to SCC in 316 steel welds
Chloride corrosion agents in Stress Corrosion
Chloride ions play a major role in the activation of SCC. In 316 stainless steel, which contains molybdenum to improve its resistance to pitting corrosion, protection can break down in the presence of high concentrations of chlorides. These ions penetrate weld imperfections or accumulate in poorly designed or undrained areas, creating favorable conditions for intergranular propagation of microcracks2.
Residual stresses and external stresses after welding
The welding of 316 stainless steel components generates significant thermal changes that leave residual stresses trapped in the heat-affected zone. When these stresses coincide with external stresses during service (internal pressure, vibrations, mechanical loads), an environment conducive to crack nucleation and crack advancement is created. This phenomenon is particularly prevalent in components such as pressure vessels, heat exchangers and piping systems, where loads and chemical conditions are constantly present.
Temperature in deterioration and cracking in 316
Although SCC can initiate at room temperature under very aggressive conditions, it is much more common at temperatures above 60 °C (140 °F). At these temperatures, ionic mobility and electrochemical reaction rate increase, accelerating the breakdown of the stainless steel passive layer and the appearance of microcracks, which can grow transgranularly or intergranularly to structural failure.
Manifestation of SCC in stainless steel welds
Welds in austenitic stainless steels, such as type 316, can become particularly susceptible to stress corrosion cracking (SCC), especially in the presence of chloride ion environments. This form of corrosion is insidious, combining metallurgical, chemical and mechanical factors that act synergistically to initiate and propagate cracks in the weld zone.
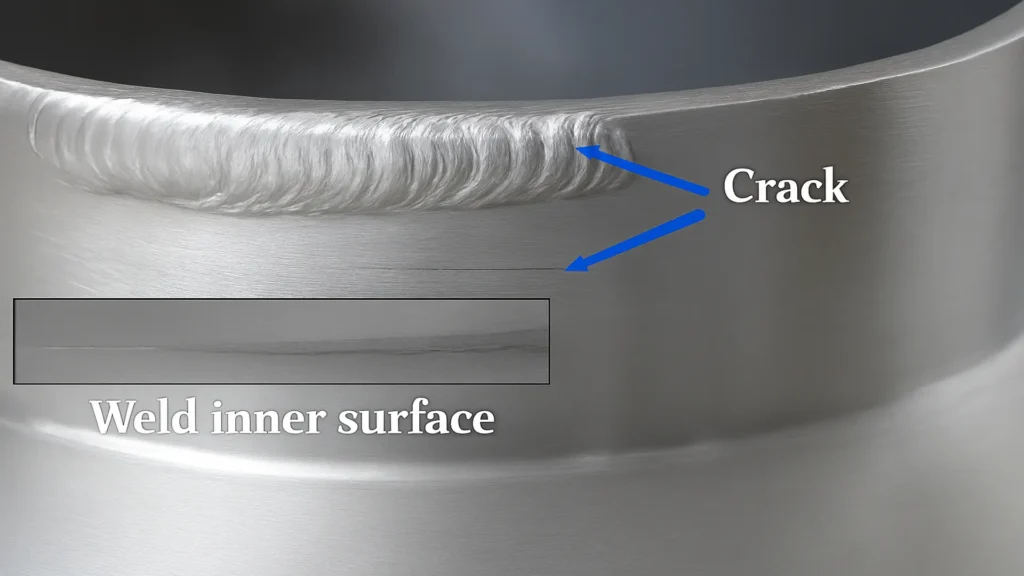
SCC in welds generally manifests itself as surface or subsurface cracks that, to the naked eye, may go unnoticed. These microcracks tend to advance slowly but steadily through the material, favored by the stress field and the action of chlorides. Their advance can be intergranular, when the crack follows the grain boundaries of the base or filler metal, or transgranular, when it passes through the grains. In both cases, the end result can be brittle failure of the component, even under normal operating stresses.
The severity of damage increases if the material is subjected to stresses above the yield stress, as occurs in U-bend tests or constant load tests. Under these conditions, SCC can rapidly progress from a difficult-to-detect incubation state to catastrophic fracture, severely compromising the integrity of the welded joint.
Recent advances in research
Several studies have shown that the regions most vulnerable to this phenomenon are usually the heat-affected zone and the molten zone, due to the microstructural alterations induced by the thermal welding process. In the specific case of 316L stainless steel, although its low carbon content reduces carbide formation and sensitization, it is still prone to SCC when residual stresses are present or applied in the presence of aggressive agents such as chlorides.
Research such as that of Garcia et al. and Kim et al. has indicated that the 316L the heat affected areascan exhibit pitting as an initial step towards SCC. This is aggravated by the possible presence of phases such as -ferrite, which can undergo selective dissolution. These pits act as initiation sites for stress cracks3,4.
Other studies have also shown that susceptibility under SCC increases with increasing temperature under deaerated conditions and with dissolved hydrogen from 0.014 to 1.4 ppm using the slow strain rate test (SSRT) method5.
In this context, it can be stated that the heterogeneous microstructure of the welded zone, combined with residual stresses and prolonged exposure to corrosive media, creates an ideal environment for SCC to manifest itself. Long-term studies, such as those conducted at 60 °C with 1.0 M NaCl solutions at pH 4, have provided evidence of how this interaction can develop under conditions that simulate real operating environments.
Finally, although accelerated tests under anodic current or extreme conditions help to evaluate susceptibility, the results must be interpreted carefully, as they do not always accurately reflect in-service behavior. Nevertheless, they are useful for identifying failure trends and critical zones within the weld of 316 stainless steel against SCC.
Prevention of SCC in 316 stainless steel welds
To mitigate SCC, multiple levels of prevention must be considered. First, proper material selection is key. Opting for 316L, or even alloys with higher crack resistance, such as duplex or super austenitic steels, can be a wise decision depending on the operating environment.
Control of the welding process is equally important. Avoiding overheating, applying qualified welding procedures and using compatible filler materials can minimize defects and stresses. In addition, whenever possible, post-weld treatments are recommended to eliminate or reduce residual stresses.
At the design level, stress concentrators should be avoided, liquid drainage should be allowed, and accessibility for inspection should be ensured. Finally, keeping under control the operating parameters: temperature, pH, chloride concentration; contributes to reduce the combination of mechanical load and chemical attack that triggers this type of corrosion.
It is important to consider the critical zones in a 316 stainless steel welded joint, which represent the areas most susceptible to SCC in a welded joint are the heat-affected zone and the edge of the weld bead. These areas concentrate high residual stresses and can exhibit sensitized microstructures, especially if critical temperatures have been reached during welding. Periodic inspections with non-destructive techniques should focus on these regions, where more than 80% of SCC cracks have been shown to initiate.
Conclusions
The formation of carbides during the welding process can significantly compromise the strength of 316 stainless steel, making the welded zone vulnerable to stress corrosion cracking. This sensitization due to the precipitation of chromium carbides at the grain boundaries reduces the protective capacity of the material against corrosive agents such as chlorides. To mitigate this risk, it is essential to opt for low carbon stainless steels, such as 316L, and to apply carefully controlled welding procedures that include adequate preheating and cooling. These measures are important to preserve the structural integrity and durability of welded components in critical environments.
Stress corrosion cracking represents a significant threat to the reliability of critical structures constructed of 316 stainless steel. This phenomenon can lead to sudden failures if an adequate prevention method is not implemented. The combination of residual stresses, aggressive agents such as chlorides and elevated thermal conditions make SCC one of the most dangerous forms of corrosion in industrial environments.
Adopting science-based approaches, advanced inspection technology and good design and maintenance practices is essential to ensure the long-term integrity of welded components. Prevention must be integrated from the design stage through to operation under a holistic view of corrosion management.
References
- Krivonosova, E. (2018). A review of stress corrosion cracking of welded stainless steels. Open Access Library Journal, 5, 1–41. https://doi.org/10.4236/oalib.1104568
- Kim, Y.-S., & Pak, S. J. (2020). Stress corrosion cracking behaviour of cold worked AISI 316L stainless steel in chloride environment. Novel Research in Sciences, 4(4). Publicado el 19 de agosto de 2020.
- García-García, V., Reyes-Calderón, F., Frasco-García, O. D., & Alcantar-Modragón, N. (2022). Mechanical behavior of austenitic stainless-steel welds with variable content of δ-ferrite in the heat-affected zone. Engineering Failure Analysis, 140, 106618. https://doi.org/10.1016/j.engfailanal.2022.106618
- Kim, S., Ahn, K., Kim, G., & Song, S.-W. (2023). Synchrotron X-ray fluorescence imaging study on chloride-induced stress corrosion cracking behavior of austenitic stainless steel welds via selective corrosion of δ-ferrite. Corrosion Science, 218, 111176. https://doi.org/10.1016/j.corsci.2023.111176
- Huang, Y.-J., & Kimura, A. (2018). Stress corrosion cracking behavior of Type 316L and Type 310S stainless steels in fusion relevant environments. Materials Transactions, 59(8), 1267–1274. https://doi.org/10.2320/matertrans.M2018064 J-STAGE
FAQs about SCC on welded 316 stainless steel
What is stress corrosion cracking?
Stress corrosion cracking (SCC) is a degradation mechanism that occurs when a metal is simultaneously subjected to a corrosive environment and tensile stresses (internal or external), which can generate cracks that progress subcritically to component failure. It is especially dangerous because it can occur without significant loss of material, making early detection difficult.
What role do chlorides play in this process?
Chloride ions readily penetrate the passive films of stainless steel and generate active sites of localized corrosion. In the presence of stresses, these sites become focal points for SCC crack initiation and propagation. The combination of elevated temperature, chlorides, and stresses makes this type of environment highly aggressive for austenitic stainless steels such as 316.
How can SCC risks be reduced?
The most effective strategies to reduce SCC include: Employing low-carbon stainless steels, such as 316L, which minimize chromium carbide formation and sensitization. Control welding procedures by preheating and slow cooling to reduce thermal stresses and prevent the material from remaining in critical temperature ranges. Avoid exposure to chloride-rich environments, or use protective coatings and corrosion inhibitors. Perform post-weld heat treatments when feasible to relieve residual stresses.
What types of inspections can detect corrosion?
The most effective methods include: Advanced ultrasound (Phased Array or TOFD) to detect internal cracks. Acoustic emission, which allows capturing crack propagation activity in real time. Eddy current inspection techniques, useful in conductive materials. Liquid penetrant (PT) and magnetic particle (MT) testing, to detect surface cracks, although limited to certain locations. Remote visual inspection (with videoscopes) and follow-up through predictive monitoring in critical areas.

