Author. Ph.D. Yolanda Reyes, February 28, 2022.
Introduction
Hydrogen currently represents the great energy alternative of the future. In this context, some companies in the automotive industry have decided to concentrate their efforts on developing this technology in order to replace fossil fuels such as gasoline and diesel and reduce pollutant emissions from the automotive industry into the environment.
However, the lack of information about hydrogen has sparked all kinds of myths around it, unlike the electric alternative, which is captivating worldwide interest in this type of vehicle. Hydrogen vehicles continue to generate certain misgivings in relation to the following aspects: from their profitability to their safety, the little information about them and their scarce presence in the current market has generated a series of myths about them, but how much truth is there? behind these vehicles?
How is the hydrogen vehicle
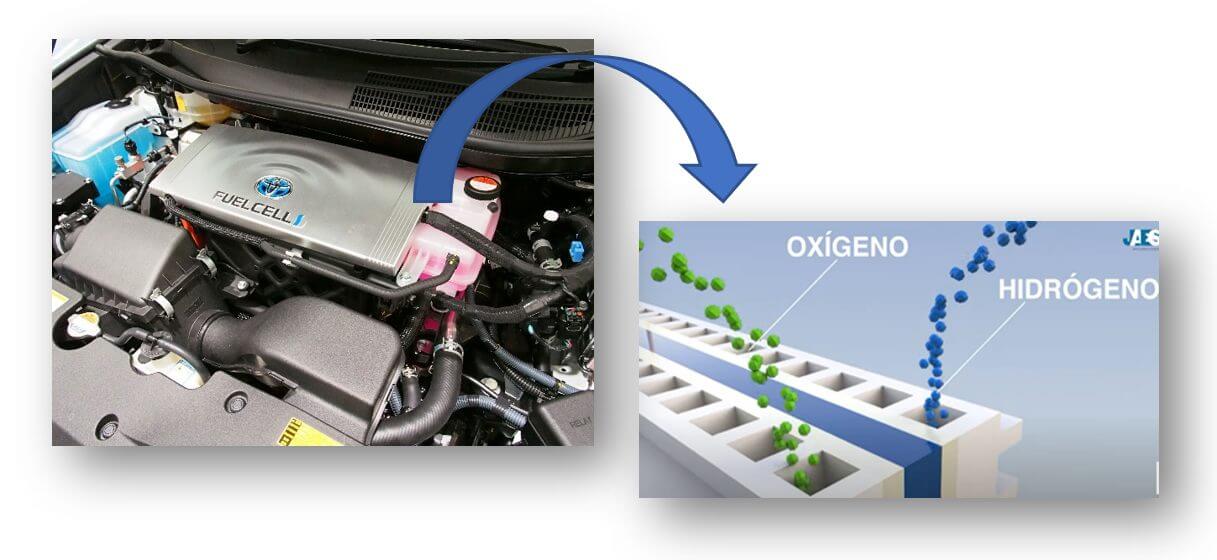
A hydrogen car is a vehicle with an electric motor that generates electricity through the chemical reaction that occurs in a fuel cell in which hydrogen and oxygen are mixed (Figure 1). Similar to electric vehicles, the differences between the two lie in the fact that hydrogen cars only take five minutes to refuel and that they have a range comparable to that of conventional models, due to the fact that they have a battery to store energy. and not to power the vehicle.
Myths about the hydrogen car
Hydrogen is currently one of the cleanest and most environmentally friendly fuels; however, there is great controversy regarding the use of this technology, qualifying it as a dangerous, expensive and polluting fluid, expressions totally unrealistic. Below are the most outstanding myths regarding the use of these vehicles.
- danger
Dangerousness is probably the biggest concern around these vehicles. It is generally thought that hydrogen storage can cause explosions; however, this gas has a much lower risk of explosion than other conventional fuels thanks to its volatility, causing it to dissipate in the event of a leak, without reaching the concentration necessary to detonate. Hydrogen has a very wide degree of flammability and a much lower risk of explosion than other fuels used, it becomes reactive between 18.3% and 59% concentration in the air; while gasoline only needs 1% to explode. Furthermore, hydrogen is a gas that rises and disperses, while propane or gasoline vapors accumulate on the ground. However, to certify reliability, these vehicles are subjected to different safety tests in which the deposits are burned, exposed to acid and high temperatures in order to ensure that accidents do not occur.
- Contamination
Hydrogen is a colorless, odorless and tasteless gas and one of the most abundant chemical elements in nature. Unlike oil, which is a primary source, hydrogen can be produced from renewable energies such as wind, solar or hydraulic, so it does not generate any type of polluting emission. On the contrary, they take polluted air from the outside to work and in return, they only expel water vapour, so their impact on the environment is positive.
- They are very expensive
Finally there is a third myth around these vehicles; which, is referred to the high costs of hydrogen fuel cells. But, although it is true that batteries currently have a high price, it is important to consider that we are dealing with an incipient technology with high production costs. The same thing happened, at first with the first vehicles: everything new is expensive; however, the passage of time will inevitably bring with it new production methods that will reduce costs by achieving; that they are accessible (Figure 2).

Is refueling safe?
Hydrogen is a very light chemical element that, under normal conditions, is colorless, odorless and does not pollute. In this article we explain the different ways of producing this fuel, a technology that is not harmful to the environment. The liquid expelled by fuel cell vehicles is the water vapor that arises from the production of electricity that ends up condensed and is emitted into the environment.
This security is also transferred to the time of fuel storage and replenishment. The storage of hydrogen involves a lot of volume and the most used technique for its conservation is in the form of gas. It is stored at very low pressure and is the most economical way to date, along with the liquid state which implies very low temperatures and superior insulation.
Unlike an electric car, a fuel cell car does not recharge using a plug. Instead, it has hydrogen tanks that mix this gas with oxygen to generate the vehicle’s propulsion. The charging time is, without a doubt, the weak point of electric vehicles. Fuel cell powered cars don’t have that problem. Its recharge is carried out in an almost identical way to that of a gasoline and diesel one, by means of a hose coupled securely and without leaks (Figure 3).

Conclution
Myth or reality, hydrogen in the not too distant future represents a source of clean and infinite energy, so it would not be affected by economic fluctuations depending on its supply and processing. The use of hydrogen as a fuel with zero CO 2 emissions, since it only expels water after its use as a propulsion method, characterizes it as a technology with high energy potential in the field of mobility, replacing fossil fuels.
References
1 – Electrosynthesis and Electrodialysis, José Ramón O Gómez.
2. Fundamentals of Electrodics, José M Acosta.
About the Author

ing Chemical Ph.D, in Electrochemistry and Corrosion, with more than 30 years of experience and a wide and versatile knowledge in Corrosion Sciences and Chemical Technology at the Academic and Industrial level. Researcher in the development of research work on Innovation and development of Electroactive Polymeric Coatings, evaluation of coatings and corrosion inhibitors and failure analysis of metallic materials. Advisor of plans of maintenance programs in the industry in oil companies, in charge of chemical and mechanical cleaning service works of equipment such as: Boilers, Heat exchangers, among others. Read more

