Summary
This article reviews one of the latest scientific advances in the search for new materials that are currently being developed through new research aimed at finding new lightweight materials that are as resistant as steel. In this context, researchers from the Institute of Technology of the University of Massachusetts (Massachusetts Institute of Technology, with its acronym in English: MIT) [1], have managed to develop a novel polymerization procedure, through which a 2D two-dimensional polymeric plastic called 2DPA-1 has been generated, which is very thin and light, but with a hardness twice that of steel.
This type of material opens the door to a new generation of ultra-resistant coatings for possible applications in the automotive industry, mobile phones or even in new construction materials for bridges or other structures; and can be easily manufactured in large quantities.
Introduction
Research and development of new types of polymeric materials impose the need for a system that allows classifying them and studying their properties. This system is based on chemical structures, behavior against heat, mechanical properties, types of applications, production scale, or even other characteristics.
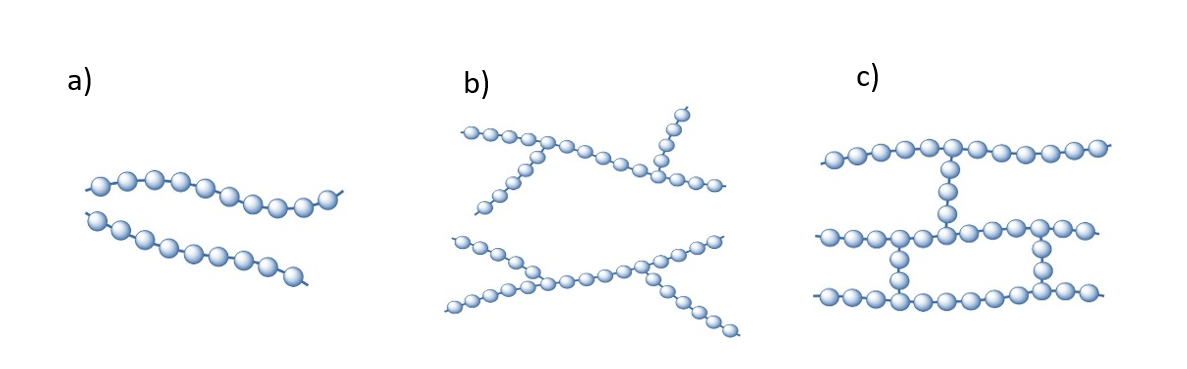
Synthetic polymers are materials widely used in different applications, with a production of more than 200 million tons per year, and are usually composed of repeating linear units. They may also be irregularly branched or intertwined (figure 1). They are organic macromolecules made up of smaller molecules called monomers.
According to the shape of the macromolecular chains, polymers can be classified as: a) Linear: they do not have ramifications, b) Branched: all molecules have branches (small side chains, c) Cross-linked: polymers have a three-dimensional structure, where the chains are linked to each other by lateral bonds.
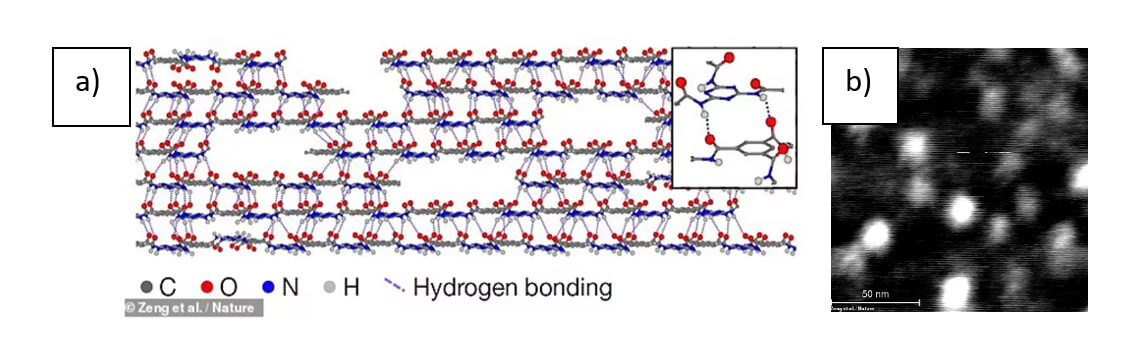
The new material developed by Yuwen Zeng. Et al [1] , at the Massachusetts Institute of Technology manages to glimpse what was believed impossible; polymerization in two dimensions. Polymerization is a process by which small atoms called “monomers” join together, usually to form long, linear chains called polymers.
Here we present a two-dimensional polymer with internal periodicity composed of repeating units arranged in the form of sheets. This is an extension of Staudinger’s concept of polymerization (to form macromolecules by covalently linking repeating units), but in two dimensions. A well-known example of such a two-dimensional polymer is graphene, but its thermolytic synthesis precludes on-demand molecular design. Here, we have rationally synthesized an ordered two-dimensional polymer that is not in equilibrium much beyond molecular dimensions. The procedure includes the crystallization of a specifically designed photoreactive monomer in a layered structure, an in-crystal photopolymerization step, and a solvent-induced delamination step that isolates individual two-dimensional polymers as independent monolayer molecular sheets.
How are these 2D polymers formed?
The key to the high resistance of these new materials is the procedure; through which they are produced[2,3] .
Until now, polymers, which include all plastics, have been generated by forming one-dimensional chains made up of blocks called monomers. These chains grow when new molecules are added to their ends and, once formed, they can be put into molds to obtain different plastic objects, such as a water bottle.
The new material, 2DPA-1, is a two-dimensional polymer that self-assembles into blocks of sheets, unlike all other polymers, which form one-dimensional monomeric chains. Until now, scientists believed that it was impossible to induce polymers to form 2D sheets.
Under the right conditions, these blocks are capable of growing in two dimensions, forming discs that are placed one on top of the other and are held together by hydrogen bonds that form between the layers. This makes the structure not only stable, but also gives the material extraordinary strength.
2D two-dimensional polymerization
According to the research carried out by these authors, two-dimensional (2D) polymers are obtained by chemical synthesis in the form of molecular sheets, using a chiral oligomeric precursor that contains two reactive sites, a polymerizable group at one end and a reactive stereogenic center near the half of the molecule[2,3] . The reaction produces 2D polymers in the form of a bilayer of molecular weight in the millions and a monodisperse thickness of 50.2 angstroms.
2D polymer molecules are formed through molecular recognition by oligomers, which self-organize into layers that place reactive groups within specific planes. Oligomers are linked by two different binding reactions involving reactive sites. At room temperature, stacks of these molecular objects can organize as single crystals and, at higher temperatures, melt into smectic liquid crystals. Nonlinear optical experiments reveal that solid films containing 2D polymers form structures that are thermally and temporally more stable than those containing analogous 1D polymers. This observation suggests that the transformation of common polymers from a 1D to a 2D architecture can produce generations of organic materials with improved properties.
Instead of building a monomeric molecule, you can make a molecular plane in which the molecules are made to hook together in two dimensions”, This mechanism occurs spontaneously in the solution, and after the material is synthesized, you can coat with ease thin films that are extraordinarily resistant.
The team found that, under the right conditions, melamine monomers can be used to grow small two-dimensional disks that stack on top of each other (as shown in Figure 2), with each layer held together by hydrogen bonds. which makes it extremely strong and stable. For a long time it was thought that polymers formed from two-dimensional sheets could be used to make extremely lightweight materials.
However, decades of research led to the conclusion that this was impossible, in part because it only takes one monomer to move out of the leaf’s growth plane for everything to lose its desired shape. However, today, thanks to the studies of these researchers, these two-dimensional polymers are self-coupling, so they can be easily produced in large quantities simply by increasing the quantities of the initial reagents.
The new material, called 2DPA-1, is known as polyaramid film and can be used to coat other materials. Unlike other polymers, whose coiled chains of monomers leave spaces between them that allow gases to leak through, the monomers in 2DPA-1 bind together tightly like bricks, making them fairly impermeable; which would make it possible to create ultra-thin coatings that completely prevent the passage of water or gases.
Strong amide-aromatic conjugation inhibits out-of-plane rotation; meanwhile, hydrogen bonding between layers […] can allow growing discs to absorb monomers from solution and automatically mold them into 2D surfaces”, Figure 3 shows the image of strong conjugation; which makes it energetically more favorable for the molecular discs to join in the same place.
Conclusion
This new discovery represents a great contribution in the development of new materials and constitutes an advanced technology in an extraordinary way, through experimentation through innovation with new components or materials. Innovation continues to drive the growing field of materials science, which in recent times has elucidated discoveries such as plastics that are harder than Kevlar or a polymer based on two-dimensional sheets that is stronger than steel.
Bibliographic references
[1] Yuwen Zeng. et al. An Irreversible Synthetic Route to an Ultra-Strong Two-Dimensional Polymer. Nature chemistry 4,. 287-291, doi:10.1038/nchem.1265 (2012) …
[2] Bresler, S., Judin, M. & Talmud, D. Mechanical properties of monolayers obtained by two-dimensional polymerization and condensation. Acta Phys. Chim. USSR XIV, 71–84 (1941).
[3] Stupp, SI, Son, S., Lin, HC & Li, LS Synthesis of two-dimensional polymers. Science 259, 59–63 (1993).

