Pulsed Current Cathodic Protection has become a highly specialized alternative for optimizing corrosion mitigation in buried pipelines, especially in scenarios where conventional cathodic protection systems, both galvanic and impressed current, fail to provide sufficient electrochemical control. Direct Current Cathodic Protection (DCCP), as a conventional technique, presents problems such as high current and energy consumption, parasitic current interference1, and cathodic detachment.
The combined use of DCCP² and (PCCP) improves overall system efficiency, optimizes current distribution, and overcomes limitations associated with deteriorated coatings, high-resistivity soils, or insufficient potential gradients. Ensuring the integrity of pipelines used for the transport and storage of petroleum derivatives requires comprehensive corrosion control methods, including coatings, cathodic protection, and continuous monitoring.
What is Pulsed Current Cathodic Protection (PCCP)?
Pulsed Current Cathodic Protection (PCCP) is an advanced electrochemical technique designed to optimize corrosion mitigation in buried or submerged metallic structures when conventional continuous current methods (DCCP/ICCP) do not achieve adequate control.
According to studies, PCCP provides greater corrosion mitigation control³. It applies controlled current pulses in terms of frequency and duty cycle to modify surface polarization, reduce effective resistance to current flow, and improve the penetration of protective potential in areas with coating defects or high-resistivity soils. Its implementation requires an integrated approach combining electrical design, electrochemical modeling, and real-time operational parameter monitoring.
How does PCCP operate and what variables influence it?
This control method operates based on the principle of cathodic protection (CP) pulse potentials to optimize current distribution in a pipeline, particularly in the presence of coating defects. The principle relies on strategically manipulating concentration polarization during the on and off cycles of the pulsed current.
Figure 1 schematically illustrates the current distribution in a pipeline with coating defects under pulsed CP potential during the on and off periods⁴. During the on cycles, cations accumulate on the steel surface, generating electrolyte concentration polarization.
Conversely, during off cycles, cations disperse from the surface, as CP is inactive during this interval. Consequently, the surface experiences temporary depolarization, eliminating concentration polarization and allowing greater CP current penetration into the steel surface. These two phenomena resemble the charging and discharging of a capacitor during the on and off cycles of CP.
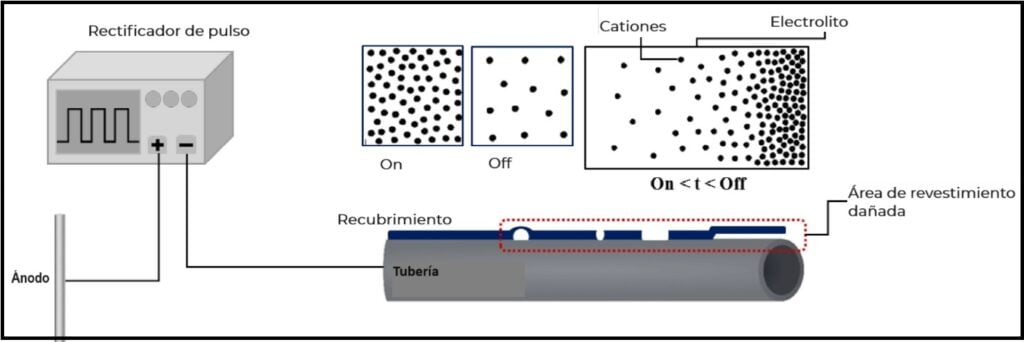
In general terms, this process achieves reduced polarization resistance and increased penetration depth of the protective current. This outcome is explained by the fact that optimal CP potential distribution is achieved at specific duty cycle and frequency levels.
Technical advantages and challenges of PCCP compared to DCCP
The performance of a DCCP system, designed to mitigate corrosion in metallic structures, can be affected by a series of external and internal factors⁵. However, PCCP offers concrete operational advantages over stationary direct current: it improves current distribution uniformity in areas with defective coating, increases the effective influence depth of protective potential, and reduces average current demand, potentially lowering anode bed requirements and energy consumption.
The pulsed nature also reduces sensitivity to stray currents induced by external sources by interrupting continuous coupling, and the OFF intervals facilitate partial hydrogen dissipation, decreasing the likelihood of cathodic disbondment and sustained overprotection. Nevertheless, PCCP presents relevant technical challenges: defining optimal parameters (frequency, duty cycle, and amplitude) depends on the environment and requires in situ characterization; stray current interference is not completely eliminated and requires monitoring and compensation measures.
Electronic models must integrate with electrochemical models to predict long-term behavior; standardization of design, testing, and acceptance procedures is still under development. Moreover, full-scale validation requires field campaigns instrumented to record potential profiles, protection currents, and soil parameters over time to evaluate durability, effects on coatings, and maintenance requirements.
Current research and challenges in PCCP
The (PCCP) represents an advanced alternative to conventional continuous current systems, challenging traditional approaches in corrosion mitigation. Recent research has intensified analysis of its electrochemical behavior to understand mechanisms that enhance current distribution and optimize protective polarization in buried structures.
Comparative studies show that high-frequency electric pulses applied in a controlled manner can generate more uniform current distribution, greater penetration of protective potential, and reduced current consumption compared to continuous en DCCP. However, comprehensive understanding of the relationship between frequency, duty cycle, and performance under partially disbonded coatings still requires consolidation, as many analyses have focused only on the electronic circuit, without fully integrating associated electrochemical effects.
Despite its potential, PCCP presents key technical challenges. Stray current interference remains a significant issue in complex industrial installations, where researchers evaluate mitigation methods to prevent external disturbances. Additionally, determining the optimal frequency-duty cycle ratio is essential to maximize system efficiency without increasing energy consumption. Another critical aspect is understanding long-term effects, including polarization stability, resistance to environmental variations, and preservation of structural integrity during continuous operation.
Future perspectives
The evolution of PCCP will depend on integrating electrochemical studies, advanced modeling, and field validation. Its widespread adoption requires overcoming current challenges through collaboration between industry, academia, and regulatory bodies. Collectively, these research efforts point toward a future where PCCP represents a more efficient, sustainable, and adaptable protection method against the growing challenges of corrosion in critical infrastructure.
Conclusion
Pulsed Current Cathodic Protection (PCCP) is a robust technical solution for cases where traditional DCCP is insufficient, providing better potential profiles, deeper protection penetration, and energy efficiency when parameters are selected based on integrated models and field data. Industrial adoption requires convergence of electrical design, electrochemical modeling, instrumented pilot testing, and regulatory frameworks to ensure replicability and performance assurance across different operational regimes.
References
- Ormellese, S., Beretta, M., Brugnett, F., Brenna, A.; “Effects of non-stationary stray current on carbon steel buried pipelines under cathodic protection,” Constr. Build. Mater., 281 (2021), Article 122645
- Shigeru, A. et al.; “Manual de electroquímica,” ScienceDirect, 2007, pp. xix-xx
- Heuze, B.; A new cathodic protection technique based on adjusting electricity to potential, in Proceedings of the First International Conference on Metallic Corrosion, London 2023, p. 394
- Afghani, M. et al.; “Investigation on utilizing pulse CP in a city gas station: A comparison with conventional CP,” Journal of Pipeline Science and Engineering, June 2023, 100109
- Barnes, J.R., Bein, R., Doniguian, T.M.; “Measurement of corrosion under disbanded coating,” Corrosion, San Antonio 2020, pp. 25-30
Frequently Asked Questions (FAQ) about PCCP
What is PCCP?
PCCP is a corrosion mitigation technique that applies high-frequency pulsed current instead of continuous current. This modulation improves protective potential distribution and optimizes steel polarization, even in pipelines with deteriorated coatings.
How does PCCP differ from DCCP?
PCCP uses controlled on/off pulses that reduce polarization resistance and allow deeper current penetration. DCCP applies stable continuous current, which can lead to high energy consumption, stray current interference, and the risk of overprotection in certain scenarios.
What are the main benefits of PCCP?
Key advantages include improved current distribution, lower energy consumption, higher efficiency under degraded coatings, and more effective mitigation in high-resistivity soil environments.
Does PCCP require replacing existing cathodic protection systems?
Not necessarily. PCCP can be implemented as a complement to conventional systems, improving performance when the existing system fails to fully polarize the structure.
What is the current state of research on PCCP?
Research focuses on correlations between frequency, duty cycle, electrochemical behavior, and field performance. Methods to mitigate interference and evaluate long-term stability are also being studied.
Does PCCP have specific regulations or standards?
Currently, there are no standards dedicated exclusively to PCCP. However, it is evaluated within existing cathodic protection frameworks, such as NACE, ISO, and DNV, and according to polarization criteria applicable to continuous current systems.

