In recent decades, the industry has witnessed constant technological innovation in the field of anticorrosive coatings, which are essential for mitigating the effects of corrosion on metal structures exposed to marine environments. These advances not only seek to improve corrosion resistance, but also to offer sustainable solutions adapted to the operational challenges of offshore and coastal areas. This article discusses the main scientific developments and trends that are transforming maritime structure protection strategies.
Fundamentals of corrosion in marine environments
Corrosion in marine environments is conditioned by specific factors, such as water temperature, salinity, dissolved oxygen content, current velocity, and the presence of chlorides. These factors vary depending on the location and depth of the structures, directly influencing the severity of the corrosive attack. The protection of metal structures exposed to these types of environments requires an integrated strategy that includes material selection, structural design, coating application, and electrochemical protection methods.
This type of damage is characterized as an electrochemical phenomenon that causes generally uniform wear, to which iron and its alloys are highly susceptible. It is generated by a natural mechanism and occurs spontaneously, destroying or reducing the structural properties of materials in service. When exposed to the elements, these materials undergo oxidation on their surface, mainly forming iron oxide1,(Figure 1).

Protection against corrosion of metal structures exposed to marine environments, whether offshore or onshore, is crucial and must be taken into account during the design of these structures to ensure they can withstand the harshness of this type of environment. Most of these structures are protected with coatings. There is no single method to prevent the different mechanisms; it cannot be avoided, but it can be controlled by manipulating the material, the metal-solution interface, or the corrosive medium.
What is an anticorrosion coating?
An anticorrosive coatings is a protective layer applied to metal surfaces to prevent or reduce the harmful effects of corrosion. These coatings act as physical barriers that prevent direct contact between the metal and corrosive agents present in the environment, such as oxygen, water, chlorides, or chemical contaminants. They can be composed of polymers, resins, metals, or hybrid materials, and their formulation is adjusted according to the conditions of the environment in which they will be used.
How do anticorrosive coatings work?
Anti-corrosion mechanism
A new generation of anticorrosive coatingthat possess passive matrix functionality and actively respond to changes in the local environment has sparked great interest among materials scientists. Corrosion is one of the most significant destructive processes involved in material loss, and its prevention is essential for protecting investments.
Active protection aims to restore the properties of the material (functionality) when the passive coating matrix is broken and corrosion of the substrate has begun. The coating must release the active, repairing material shortly after the integrity of the coating has been compromised. This acts as a local trigger for the mechanism that heals the defect.
Figure 2 shows the mechanism of action of these types of coatings in the presence of a defect (crack) in the paint. In this case, when the damage breaks the capsule, the monomer it contains is released and reacts with the catalyst in the coating to seal the crack2,3.
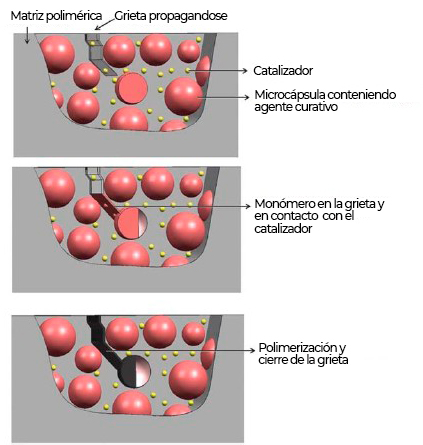 | These types of coatings are based on their ability to automatically and independently repair any type of imperfection or damage that occurs in the coating. To achieve this effect, two types of technologies are used: addition of polymeric nanocapsules or inhibiting potential corrosion areas through inhibitors. The most used method is to add microcapsules that act directly on damaged areas. These microcapsules contain some type of liquid, solid or gaseous nanoparticle, which gives rise to the repair of the coating |
Corrosion control is a requirement for all structures exposed to a saline environment. While most surfaces are designed to withstand the environment, coatings are necessary to protect them effectively.
Recent advances in anticorrosion coatings
- High-performance coatings: Designed with epoxy resins, polyurethanes, and modified phenolic polymers, these coatings provide greater adhesion, chemical resistance, and thickness. They are ideal for submerged areas or areas subject to continuous splashing.
- Nanostructured coatings: Nanotechnology has enabled the incorporation of particles such as SiO2, TiO2, metal oxides, and zinc compounds at the nanoscale. These improve the compaction of the coating, its resistance to water and contaminant diffusion, and provide self-cleaning or photocatalytic properties.
- Smart and self-repairing coatings: These include chemical sensors or capsules that release inhibitors or repair monomers when they detect a crack or degradation of the coating. They are capable of automatically restoring protective functionality in the event of localized damage.
- Friendly coatings: Formulated with water-based, solvent-free, and renewable materials. They minimize environmental impact and toxicity for workers without compromising corrosion protection.
Scientific research into anticorrosive coatings
Currently, a series of studies are being conducted focused on the development of new anti-corrosion methods for marine structures. The aim is to determine the most appropriate control method to protect these structures in an environment as aggressive as the marine atmosphere.
The most innovative studies in this field of corrosion are outlined below:
CTC Component Technology Center, in Catambria, Spain
Based on its findings, this Technology Center has developed a wide variety of innovative paints and coatings that improve their anti-corrosion properties in marine environments that cause damage to metal structures.4.
NANOMAR Project (EU-Brazil-Russia)
Develops smart bifunctional coatings with anti-fouling and self-repairing properties for offshore wind farms. They use nanocontainers that release biocides and inhibitors in response to damage5.
Polymer nanocomposites
Studies show that introducing nanoscale inorganic materials (such as clays, metal oxides, and graphene) into polymer matrices significantly increases resistance to corrosion, UV rays, mechanical impacts, and thermal aging6.
Applications of coatings in marine environments
Offshore platforms in the oil and gas industry
When it comes to corrosion protection, operations in the oil and gas industry require substantial capital investment. The areas comprising the splash zones of offshore platforms face severe conditions, both in terms of their exposure to ultraviolet radiationand constant cycles of moisture and drying, as well as the impact and abrasion caused by floating debris, hurricanes, and even floating ice sheets5.
If left unpainted, the corrosion rate of steel in splash zones would easily exceed 250 microns per year. This means that steel must be protected, with paint being the most widely used method of protection.
anticorrosive coatings systems usually consist of several layers that form a barrier against the penetration of water and contaminants through the coatings applied to steel. However, the properties of coating systems can only be fully realized if they have been properly applied and if the surface has been optimally prepared beforehand. It has been widely demonstrated that the quality of surface preparation has a direct relationship with the service life of a system.
Getting it right from the outset is paramount in the oil and gas industry, as both access and timing are obstacles when it comes to applying maintenance paint systems.
Currently, “The NANOMAR project aims to establish a lasting scientific collaboration network between European research institutions and scientists from two BRIC countries, namely Brazil and the Russian Federation. In the search for the development of new “sustainable smart materials” for offshore applications (Figure 3), in the development of new functional nanocontainers, new protective coatings, as well as equipment with recognized expertise in the characterization of anticorrosive coatings.

The main scientific objective of the proposal is to develop a new generation of “smart” bifunctional coatings that combine self-repairing anti-corrosion capabilities with anti-fouling properties for offshore applications, such as oil rigs and wind farms.
The main scientific approach underlying this project is the controlled release of active species (corrosion inhibitor and biocide agent, respectively) from nanostructured containers (nanocontainers) in damaged areas of the coating.
Offshore wind farms
One of the greatest challenges for the 21st century is to ensure energy supply with a focus on renewable energy sources. Smart anti-corrosion and anti-fouling coatings have been developed that can withstand the harsh conditions of the marine environment and will therefore help to meet these objectives.
Offshore wind farms (Figure 4) are one of the most promising technologies for meeting global energy demand in a sustainable manner. The EU supported the development of smart materials to reduce damage and improve protection against fouling of marine structures through funding for the project “Nanocontainer-based active coatings for maritime applications” (NANOMAR).
Nanocontainers are tiny compartments that store molecules for controlled release. Their use in coatings to release biocides and corrosion inhibitors in response to damage to the coating has been investigated. The first step was to integrate each of the biocides or corrosion inhibitors into coatings to test their individual performance in controlled experiments.

The team used electrochemical techniques and accelerated corrosion resistance tests and anti-fouling capacity assessments during exposure to offshore platforms. The most promising nanoadditives were mixed into a multifunctional coating and tested in operation on an offshore platform in Brazil.
Conclusions
The development of advanced anti-corrosive coatings represents an essential solution for the protection of metal structures in marine environments. Emerging technologies, such as smart, nanostructured, and eco-friendly coatings, together with proper application and planning, make it possible to extend the useful life of these structures, reduce maintenance costs, and ensure their integrity. The synergy between scientific research and industry will continue to be key to addressing challenges in the maritime sector.
Studies are currently being conducted to select the most suitable multifunctional system for industrial implementation. The development of a new generation of smart and sustainable coatings will benefit numerous sectors. Among these, the energy and maritime transport sectors stand out as true pillars of the economy and fundamental activities for global socioeconomic progress.
References
- (NACE, 1981, Uhlig, 1973).
- https://ingenieromarino.com/recubrimientos-basados-en-nanoparticulas-para-aplicaciones-offshore.
- Abdel and M. Madkour, “Potential use of smart coatings for corrosion protection of metals and alloys” A review,” J. Mol. Liq., vol. 253, pp. 11–22, 2018.
- Grinon Rosa, https://centrotecnologicoctc.com/2021/10/20/investigacion-e-innovacion-ganar-la-batalla-la-corrosion/
- https://ciencia.estudiareneuropa.eu/s/3971/76707-Ingenieria-industria/4054530-Recubrimientos-inteligentes-para-estructuras-maritimas.htm?c1=26254&l=90
- Tian, Y., Huang, H., Wang, W., Ma, Y., He, X., Zhang, L., Sheng, X., & Zhang, X. (2023). Recubrimientos a base de polímeros a nanoescala resistentes a la corrosión. En Materiales a nanoescala basados en polímeros para recubrimientos de superficies (pp. 547–584). Elsevier. https://doi.org/10.1016/B978-0-323-85190-2.00009-4

