Table of Contents
Hydrogen is increasingly recognized as a cornerstone of the energy transition, with the potential to decarbonize sectors that are difficult to electrify, such as heavy industry, aviation and long-distance transport. As an adaptable energy carrier, hydrogen can be produced from renewable sources, offering an opportunity to reduce greenhouse gas emissions and contribute to global climate goals. However, significant barriers remain to realizing hydrogen’s full potential, a major one being the logistics of its large-scale production, storage and transportation.
By 2050, global spending on hydrogen-to-energy production is expected to reach $6.8 trillion, with an additional $710 billion earmarked for ammonia pipelines and terminals. Supply chain logistics remains a key challenge. As investments grow, pipelines are seen as the most cost-effective method of transporting hydrogen. In the United States alone, three million miles of natural gas pipelines and more than 1,600 dedicated pipelines are expected to be tapped.
Mixing hydrogen with natural gas allows reuse of existing pipelines, but the properties of hydrogen present challenges such as material incompatibility, embrittlement and loss of ductility. One study reported a 60% reduction in the ductility of existing pipeline materials.
Pipe coatings are widely used to prevent corrosion by creating a barrier between the pipe material and hydrogen. However, these coatings present limitations such as compatibility issues with hydrogen and other chemicals, diffusion leading to embrittlement, and the risk of material failure due to coating defects.
Despite the urgent need for solutions to support the hydrogen economy, research on the use of chemical corrosion inhibitors, commonly employed in the oil and gas industry to mitigate corrosion, has been minimal. As emissions targets are tightened, demand for hydrogen-compatible chemicals is expected to increase. Products such as those used in natural gas production and transportation (e.g., corrosion inhibitors, hydrate inhibitors, anti-caking agents, antifoulants, amines, and hydrogen sulfide scavengers) will also be needed for hydrogen-blended gas.
ChampionX has conducted compatibility tests to evaluate the performance of its chemical products, revealing that some products with a long history of stability when subjected to traditional tests showed instability in the presence of hydrogen.
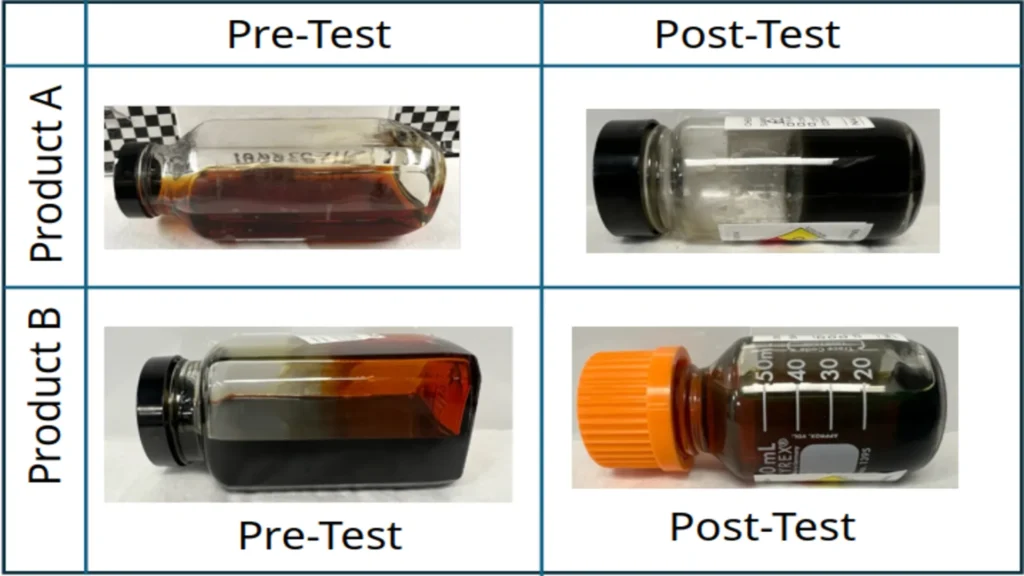
The figure below shows the results of two different chemicals tested before and after exposure to hydrogen during a residue test. For Product A, unwanted solids and a sticky residue formed, which could have a direct impact on piping or compressors by causing product buildup.
ChampionX also tested API X65 steel (UNS K03014) by cathodic charging in an acid solution to evaluate resistance to hydrogen absorption in the presence of various corrosion inhibitors (once compatible chemicals were identified). The results suggest that the presence of corrosion inhibitors creates a barrier against hydrogen adsorption. However, the selection of appropriate inhibitors requires careful consideration, as not all inhibitors offer maximum protection.
In this study, INH-1 (a water-insoluble inhibitor) showed the best performance compared to INH-2 (a water-soluble inhibitor). Resistive polarization (Rp) values, obtained by electrochemical impedance spectroscopy (EIS) after loading, increased significantly for all potentials compared to both control and INH-1 solutions. The values ranged from 302,300 ohm-cm² to 190,700 ohm-cm², compared to 5,179 and 61 for INH-1. These high Rp values for INH-2 indicate the formation of an excellent barrier against hydrogen penetration, as it formed a persistent and continuous layer that prevented hydrogen atoms from penetrating the metal.
Opportunities exist to further investigate corrosion inhibitors to take advantage of hydrogen infrastructure, comprised of existing pipelines for transport. While identifying suitable metallurgy for new infrastructure is ideal, reusing existing assets offers significant cost benefits, especially since many oil and gas pipelines are already equipped to inject and monitor these chemicals.
If hydrogen adoption accelerates as expected, corrosion inhibitors can be effectively implemented and monitored through robust inspection programs. Collaborating with pipeline operators will be essential to validate the safety and efficacy of these solutions, while careful consideration of existing inhibitors is necessary to avoid compatibility issues.
Overall, chemical corrosion inhibitors are a promising avenue to support the large-scale adoption of hydrogen, but investment in research and development is crucial to ensure safe and sustainable transportation.
References
- https://www.dnv.com/focus-areas/hydrogen/forecast-to-2050/
- U.S. Department of Energy, “HyBlend: Opportunities for Hydrogen Blending in Natural Gas Pipelines,” Hydrogen and Fuel Cell Technology Office, December 2022.
- Hardie D, Charles EA, Lopex AH, Hydrogen Embrittlement of High Strength Pipeline Steels, Corrosion Science, Vol 48, pp. 4378-4385, 2006.
- Caleb Clark, Geeta Rana, Ana Ferrer, Yolanda De-Abreu, Matthew Trevino, Jeremy Bartels, Chemistry Matters – Hydrogen Compatibility with Gas Production Chemicals, Proceeding from 19th Pipeline Conference Technology 2023, Berlin, Germany.
This article was developed by specialist Yolanda De-Abreu and published as part of the fourth edition of Inspenet Brief magazine December 2024, dedicated to technical content in the energy and industrial sector.