A new avenue for sustainable aviation has been opened up by an international team led by researchers from the National University of Singapore (NUS). Using a nickel catalyst system, the group was able to transform carbon dioxide into branched liquid hydrocarbons, essential elements of fuels such as gasoline and kerosene for airplanes.
The nickel catalyst system
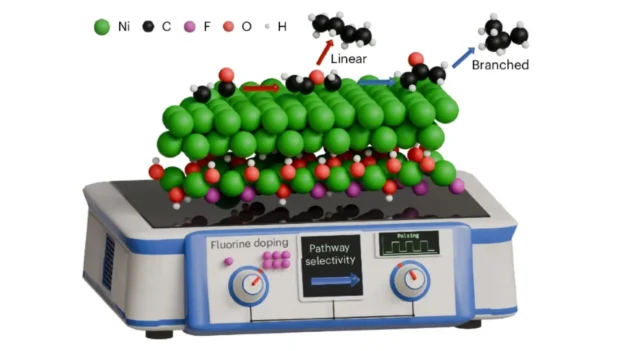
So far, copper has been the protagonist in the electrochemical reduction of CO2. However, its performance has been limited to simpler molecules such as ethanol or ethylene. The new approach of the team led by Prof. Boon Siang Yeo used fluorine-doped catalysts on a nickel base, which overcame this barrier. This technology enabled the production of longer hydrocarbons with branched structures, ideal for improving the performance of liquid fuels.
One of the keys to progress is the application of electrolysis application of pulsed potential electrolysis pulsed potential electrolysis, a technique that varies the electrical polarization in cycles. This strategy not only improved the efficiency of the process, but also increased the proportion of branched versus linear hydrocarbons by more than 400 % compared to traditional methods.
According to the team, these results are due to nickel’s ability to facilitate the removal of oxygen from CO2 intermediates, which favors the formation of complex structures. Unlike copper, which tends to generate alcohols and slows the growth of long chains, nickel directs the reaction toward more energy-dense compounds.
The work was carried out in collaboration with the Institute for Chemical Research of Catalonia (Spain) and ETH Zurich (Switzerland). The combination of catalytic synthesis, computational modeling and mechanistic analysis allowed understanding at the molecular level why nickel outperforms copper in this type of process.
The findings, published in Nature Catalysis, offer a new perspective on how to recycle CO2 efficiently, with direct potential to power sustainable aviation systems and reduce reliance on conventional high-temperature processes such as Fischer-Tropsch synthesis.
This development represents an important step in the transition to a low-emission economy. By enabling the manufacture of fuels on demand with CO2 as the main input and electricity as the energy source, the nickel catalyst platform offers a viable and scalable alternative to produce liquid energy with less environmental impact.
For Prof. Yeo, this breakthrough is the result of strategic scientific cooperation: “The dialogue between experimentation and theory allowed us to discover new catalytic pathways and move towards more efficient fuels.
Follow us on social networks and don’t miss any of our publications!
YouTube LinkedIn Facebook Instagram X (Twitter) TikTok
Source and photo: NUS