When preparing material for my corrosion classes, I like to research and find cases that are interesting and catch the attention of my students and colleagues. From here I was able to read a little about the interesting history of the Eiffel Tower, also known as The Iron Lady, a wonder of wrought iron that has exceeded its useful life by design of 20 years (It is currently 121 years old), thanks to a rigorous maintenance program that is mainly based on the replacement of corroded parts and the use of a repainting scheme with a frequency of 7 years, as a method of prevention and control of atmospheric corrosion.
Keywords: Atmospheric corrosion, inspection, maintenance, coatings, Eiffel Tower.
About the tower and my interest
This tower was built in 1889 with wrought iron in the city of Paris, France. It currently has a height of 324 meters to the tip of the antenna. For more information you can go to the page https://www.toureiffel.paris/es/el-monumento/historia. Most of the people who visit this impressive iron monument are interested in taking the best photo, day or night, showing the beauty of this monumental structure. In my case, I would also be interested in seeing the state of oxidation and/or preservation of the iron structure and analyze how a structure designed for a useful life of 20 years, today over 120 years old, can be a good reference as a case of successful prevention and control of corrosion, due to a good selection of materials for that construction period and an adequate maintenance strategy focused on the life extension of this asset.
high purity iron
The purity of the iron used in the construction of the Eiffel Tower is the result of a process in high-temperature furnaces. This process allows the carbon content to be reduced to a very low percentage and, above all, almost all the sulfur is eliminated, so the resulting iron is of high purity. It is made up of more than 18,000 pieces of wrought iron joined by means of hot rivets, this with the intention of ensuring the fixing of the pieces, since when the rivets cool, they contract and thus ensure the union of the pieces. This system must be kept in good condition to prevent corrosion in confined spaces between the rivet and the part.

I have read that some corrosion specialists have given different opinions regarding the possibility of other alternatives such as steel and/or galvanized steel and copper alloys, which may also be valid. At this point should we think, in 1889, what would it be like to make a different selection based on the knowledge of materials of that time and the risk that this would have represented?
corrosion mechanisms
Being a structure exposed to the environment, the predominant corrosion mechanism will be atmospheric corrosion (initiation and propagation), erosion due to the presence of particles and wind, and confined spaces in some areas and parts of the tower that allow the accumulation of humidity and concentration of aggressive species.
The initial corrosion reactions will be the oxidation of iron (Fe → Fe+2 + 2e−) and the reduction reaction of oxygen (1/2 O2 +H2O+2e− → 2OH−) in the moisture film formed on the surface iron, both as part of the initiation process of this mechanism. At the beginning there will be an increase in the pH in the cathode promoting the precipitation of corrosion products and from here new reactions and compounds are developed based on the aggressive species of the environment (SO2, Cl-) in what is called the propagation phase. .
The iron forging process favors the much slower dissolution of iron from the point of view of the electrochemical process, because it keeps the anodic and cathodic zones very close in terms of their electronegativity values. However, other factors come into play such as changing climatic conditions (magnitude of the wind, rain, cold, ice, solar radiation, cause the paint to blister and peel off, exposing the iron, which produces oxidation, erosion due to the presence of particles and wind speed, bird feces and pollution, the latter much more accentuated today, which will accelerate localized corrosion processes in different shapes and parts of the tower.

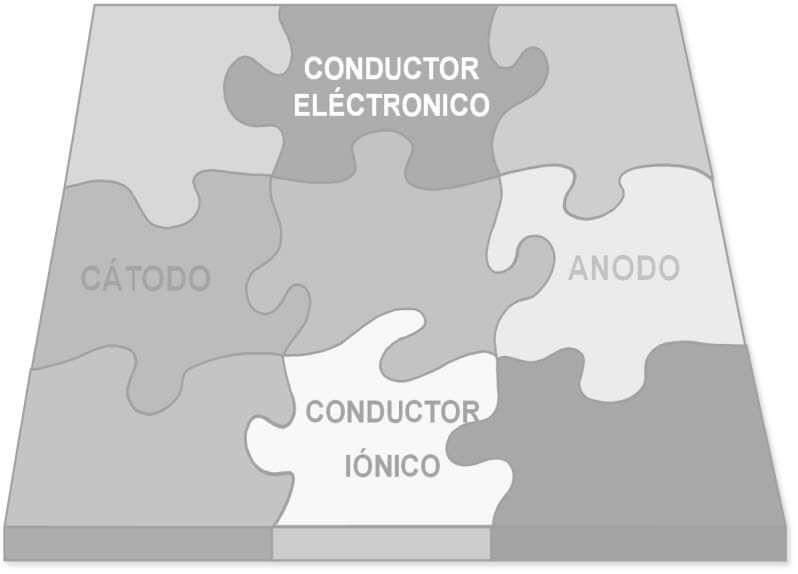
Corrosion Prevention and Control
To prevent corrosion of the metal structure of the tower, you simply have to think about the elements of the electrochemical corrosion cell: anode, cathode, ionic conductor and electrical conductor, climatic conditions and pollution. The method that has been used since its construction was designed by Gustave Eiffel himself, considering the separation of the ionic conductor (moisture and aggressive species in the atmosphere) from the rest of the elements through the application of a coating system, with a frequency of every 7 years.
The inspection and surface preparation program for parts with coating damage and surface corrosion and the manual application of 60 tons of two-layer urethanized alkyd resin-based coating. Parts with severe corrosion are replaced by new parts that are also coated. Lead coatings were still being used as recently as 2001, when the practice was discontinued for environmental reasons. Recently, new lead-free pigment coating systems have been applied, tested by the SNTE (Société Nationale de la Tour Eiffel). To emphasize the shape of the tower seen from the ground, the tower is painted in three shades of brown specified by the SNTE architect: the lighter shade at the top and the darker at the bottom.
Curiosities
For its inauguration in 1889, the Eiffel Tower was painted dark red, then ocher from 1892, yellow from 1899, mustard until 1954, maroon until 1968, and brown (bronze) ever since. Since its construction, it has been covered manually with brushes more than 19 times, that is, every 7 years a maintenance program is applied to the structure.
25 painters use 1,500 brushes and rollers to apply 60 tons of anti-corrosion coating during an 18-month renovation period. Today, the cost of these repainting works has an approximate cost of 4 million euros and the work lasts approximately 18 months (an advantage that does not require the monument to be closed to the public at any time.

Source. own
This writing was prepared by Ing. Gustavo Romero / G.romero@penspen.com. This summary is intended to be informative and not for profit, rather than to share knowledge. I hope you find it as interesting as it did for me.


